Distinguishing Between Benzyl and Phenyl Functional Groups
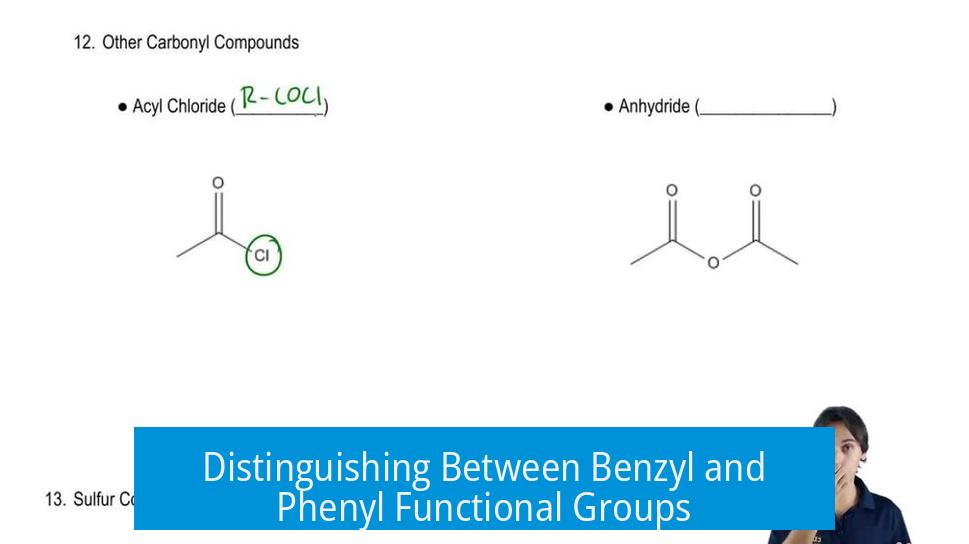
The main difference between benzyl and phenyl groups lies in their point of attachment to the parent molecule: a phenyl group is a benzene ring directly attached, whereas a benzyl group includes a methylene (-CH2-) linker between the benzene ring and the parent structure.
Definitions and Basic Structures
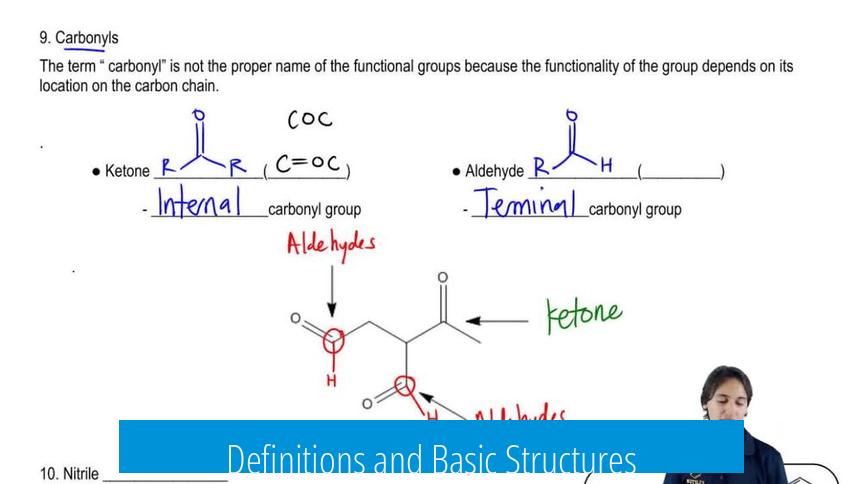
- Phenyl group (C6H5-): A benzene ring missing one hydrogen atom, connected directly to the rest of the molecule through a carbon atom on the ring (an sp2 carbon).
- Benzyl group (C6H5-CH2-): A benzene ring attached to the parent molecule via a methylene (-CH2-) group, sometimes described as a toluene-derived group minus a hydrogen from the methyl substituent.
Structural Attachment Differences
The key feature distinguishing phenyl from benzyl is whether the benzene ring bonds directly to the parent chain or if it bonds through a benzylic carbon (the -CH2- group).
| Functional Group | Attachment to Parent | Structure |
|---|---|---|
| Phenyl | Direct attachment through ring carbon (sp2) | C6H5- |
| Benzyl | Attachment via -CH2- (benzylic carbon) | C6H5-CH2- |
Practical Example: Aspartame
In aspartame’s structure, the aromatic side is a benzyl group rather than simply phenyl because the benzene ring connects to the amino acid chain through a methylene (-CH2-) group. This subtle distinction illustrates how different naming depends on the exact bonding.
Summary of Differences
- Phenyl: Benzene ring attached directly to the parent molecule without any intervening atoms.
- Benzyl: Benzene ring attached to parent via one methylene (-CH2-) carbon.
- Identifying the main carbon chain clarifies which group is present.
Key Takeaways
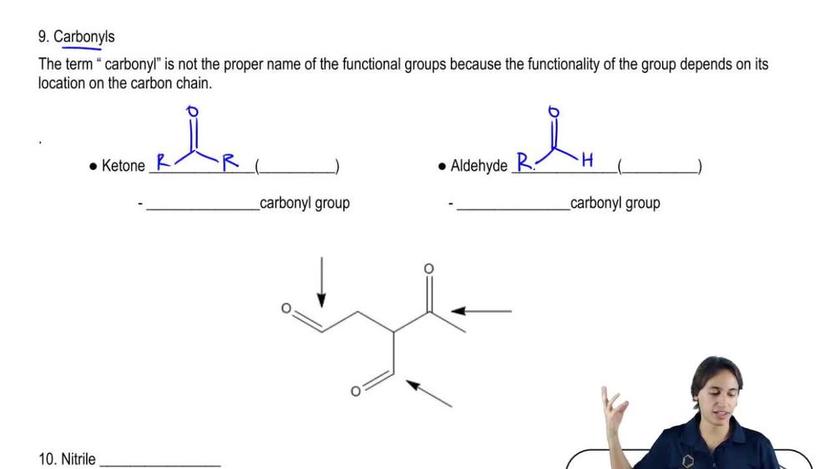
- Phenyl group has no -CH2- linker; benzyl does.
- Phenyl attaches directly to an sp2 carbon on the ring.
- Benzyl attaches via a benzylic sp3 carbon (methylene group).
- Correct naming requires identifying the bond point to the main chain.
How Do You Tell the Difference Between Benzyl and Phenyl Functional Groups?
Simply put, the benzyl group is a benzene ring attached to a -CH2- group, which then connects to the rest of the molecule, while the phenyl group is just the benzene ring attached directly to the parent structure. Let’s dive into this subtle, yet mighty distinction—because chemistry loves to play with details.
So why should you even care about telling them apart? Well, in chemistry, small differences in structure lead to big differences in reactivity, properties, and even product outcomes. Imagine mixing up your phenyl and benzyl groups in a synthesis—it’s like putting cream in your coffee instead of sugar. Both white, but completely different taste and effect.
What Exactly are Phenyl and Benzyl Groups?
The phenyl group is the simpler of the two—think of it as just the benzene ring missing one hydrogen atom. Formally, it’s C6H5-. Picture a hexagon with alternating double bonds; now remove one hydrogen to create a reactive “arm” that can attach to other parts of your molecule.
The benzyl group, on the other hand, adds a little spacer: a methylene (-CH2-) between the benzene ring and the rest of the molecule. That’s why it’s usually written as C6H5-CH2-, or “phenyl-CH2-.” You can imagine it as a little extension cord connecting the benzene ring to the rest.
Attachment Points: The Key Difference
Here’s your chemistry cheat sheet:
- If the benzene ring is attached directly to the rest of the molecule—boom, that’s phenyl.
- If there is a -CH2- group linking the benzene ring to the parent structure, you’ve got benzyl.
This distinction is crucial because the extra benzylic carbon (-CH2-) in benzyl groups imparts different chemical behaviors. For example, benzylic positions are famous in organic chemistry for their ability to stabilize carbocations and radicals. This means benzyl compounds often react differently under various conditions compared to phenyl compounds.
An Aspartame Story: Benzyl or Phenyl?
To make this more than just theoretical, consider the case of aspartame, the popular artificial sweetener. It contains an amino acid residue called phenylalanine. Now, phenylalanine’s side chain isn’t just a phenyl group stuck right on. Instead, the aromatic ring is connected via a -CH2- spacer to the amino acid backbone.
Thus, the aromatic ring in aspartame acts like a benzyl group, because there’s that bridging methylene carbon. If you were to say it’s phenyl, you’d be ignoring the -CH2- link, which is chemically important.
So here, calling it “benzyl” or “phenyl” depends on your reference point. If you focus just on the aromatic part, it’s phenyl. But when you look at its place in the whole molecule, it’s benzyl. This example shows how context matters in chemistry lingo.
Why This Matters: Practical Implications
Let’s put on our chemist hats for a moment. Knowing if you’re dealing with phenyl or benzyl affects how you predict chemical reactions. Benzyl carbons are reactive sites, especially prone to oxidation or substitution, which phenyl carbons don’t favor as much.
For instance, benzyl chloride (C6H5-CH2Cl) behaves quite differently than chlorobenzene (C6H5-Cl), despite both containing benzene rings and chlorine. The benzylic position in benzyl chloride is reactive, making it a favorite starting point for various syntheses.
This plays into pharmaceuticals, materials science, and fragrance chemistry, where the molecular structure tailors the final product’s properties and activity.
Quick Tips to Spot the Difference
- Look at the atom directly connected to your parent molecule:
- If it’s a carbon that’s part of the benzene ring itself → phenyl.
- If it’s a separate -CH2- carbon before the ring → benzyl.
- Remember, benzyl has one extra carbon atom compared to phenyl.
- Visualize: phenyl sticks straight onto the chain, benzyl “holds the ring at arm’s length” with a -CH2-.
When the Line Gets Blurry
Chemists sometimes debate terminology depending on the context. For example, in naming or mechanisms, the functional group may be simplified for ease, or viewed from different angles. Knowing the exact structural link can clarify confusion.
So if you’re reading a chemical paper or working on a synthesis, pause and identify the attachment carefully. This move helps avoid mistakes and improves understanding of the molecule’s behavior.
Summary Table: Phenyl vs. Benzyl
| Feature | Phenyl Group | Benzyl Group |
|---|---|---|
| Chemical Formula | C6H5- | C6H5-CH2- |
| Attachment Point | Directly on benzene ring carbon (sp2) | Via a methylene (-CH2-) linking carbon |
| Example | Chlorobenzene (C6H5-Cl) | Benzyl chloride (C6H5-CH2-Cl) |
| Chemical Reactivity | Less reactive at the ring-carbon site toward substitution | Benzylic carbon highly reactive and important in radical/stability chemistry |
| Biochemical Example | Phenylalanine side chain conceptual | Aspartame aromatic ring linked by -CH2- |
Wrapping It Up
Next time you scan a chemical structure, ask yourself: Is that aromatic ring glued directly on, or held at arm’s length by a -CH2- spacer? That tiny difference flips the entire functional group identity between phenyl and benzyl.
Understanding this distinction deepens your chemical intuition. It’s like recognizing the subtle difference between a direct handshake (phenyl) and the classic “fist bump” via a spacer (-CH2-) (benzyl). Both friendly, but not the same!
Got molecules to spot or reactions to run? Keep that benzylic carbon in mind—it’s the star player distinguishing benzyl chemistry from phenyl’s straightforward style. And remember, if your chemistry gets confusing, just picture the ring hanging out on an extra carbon spacer. That’s your benzyl group waving hello.
What structurally differentiates a phenyl group from a benzyl group?
A phenyl group is a benzene ring directly attached to the parent chain. Benzyl has a -CH2- group linking the benzene ring to the parent chain. This -CH2- is called the benzylic carbon.
How can the point of attachment help identify whether a group is phenyl or benzyl?
If the benzene ring bonds directly to the main structure through an sp2 carbon, it is phenyl. If the ring connects through a CH2 group, it is benzyl.
Can you give an example where the distinction between phenyl and benzyl matters?
In aspartame, the aromatic ring is linked by a -CH2- group, making it a benzyl group rather than just a phenyl group.
Is the difference between phenyl and benzyl related to the presence of the benzylic carbon?
Yes, benzyl includes the benzylic carbon (-CH2-) between the benzene ring and the main chain. Phenyl lacks this bridging carbon.
Does the functional group name depend on the parent chain in a molecule?
Yes. Phenyl or benzyl identification depends on the exact point of attachment to the parent structure, especially if the ring attaches directly or through a -CH2- group.




Leave a Comment