Understanding Why (S)-2-Iodopentane Is Called “Primary” in SN2 Reactions
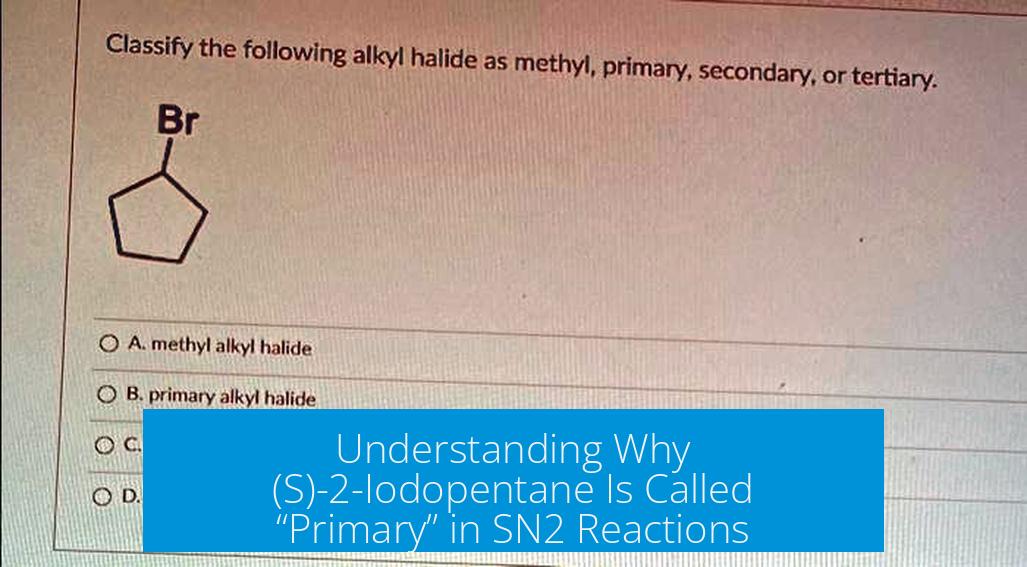
(S)-2-Iodopentane is typically classified as a secondary alkyl halide, not primary. In standard nomenclature and organic reaction mechanisms, 2-iodopentane has the iodine substituent on the second carbon, which is bonded to two other carbons, making it secondary. If your solutions manual calls it “primary,” it likely contains an error or a specific context is missing.
Primary vs Secondary Alkyl Halides
- Primary alkyl halides: Halogen attached to a carbon bonded to only one other carbon (e.g., 1-iodopentane).
- Secondary alkyl halides: Halogen attached to a carbon bonded to two other carbons (e.g., 2-iodopentane).
- Tertiary alkyl halides: Halogen attached to a carbon bonded to three other carbons.
By definition, (S)-2-iodopentane has the iodine on C-2, which connects to two carbons, classifying it as secondary. This explains why SN2 reactions typically proceed more slowly with it than with primary substrates due to steric hindrance.
SN2 Reactivity and Substrate Structure

SN2 reactions favor substrates where the nucleophile can easily attack the electrophilic carbon. Primary halides react fastest because there is minimal steric hindrance. Secondary halides react slower, and tertiary halides rarely undergo SN2.
Since (S)-2-iodopentane is secondary, its reactivity in SN2 is lower relative to a primary alkyl halide. The “(S)” indicates stereochemistry and does not change the carbon substitution level.
Possible Reasons for the Discrepancy
- Typographical error or mislabeling in the solutions manual.
- Misinterpretation of the substrate: Sometimes a substrate is drawn incorrectly or the naming was mixed.
- Context-specific definition: If the problem or manual defines “primary site” differently or focuses on a different center, but this is uncommon.
What to Do When Confused by Contradictory Sources
- Verify the molecule’s structure: Check connectivity to confirm primary vs. secondary.
- Consult reputable textbooks or trusted online resources for SN2 substrate classifications.
- Discuss with instructors or peers to clarify ambiguities in study materials.
- Be mindful of fatigue—taking breaks improves problem-solving and comprehension ability.
Key Points
- (S)-2-Iodopentane is a secondary alkyl halide based on carbon substitution.
- Primary substrates have the halogen on a carbon bonded to only one other carbon.
- SN2 reactions proceed more readily on primary halides than on secondary ones.
- If a solutions manual calls (S)-2-iodopentane “primary,” it likely contains an error or lacks clarification.
- Review molecular structures and trusted references to ensure correct classification.




Leave a Comment