Which Phenyl Ring Bears More Positive Charge?
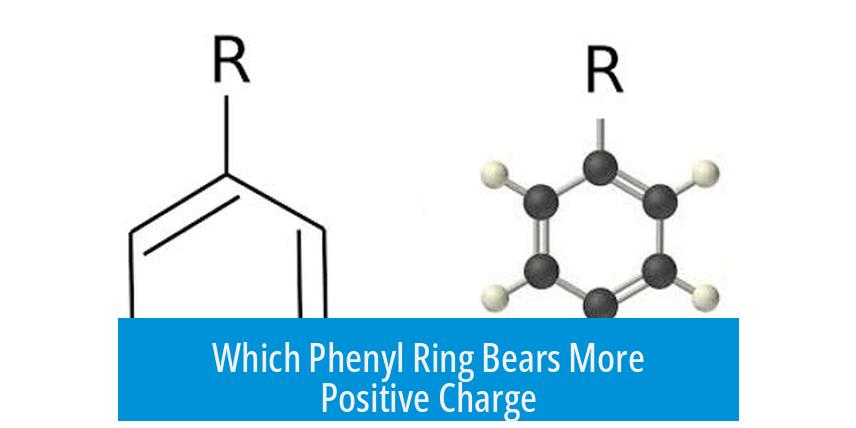
The right phenyl ring bears more of the positive charge. This stems primarily from the resonance structures involving the attached oxygen atom and the center carbon. The oxygen donates electron density toward the ring, generating a negative ring resonance form that tends to partially cancel out the positive charge on that ring. Consequently, the right ring, its central carbon, and the oxygen atom exhibit partial positive charges (δ+), while the left ring remains nearly neutral.
Resonance and Charge Distribution
The distribution of positive charge across phenyl rings depends heavily on resonance structures. In this case, resonance shows that the oxygen’s electron donation creates a negative resonance form in the right ring, which offsets some positive character. The remaining resonance contributors place the positive charge mostly on the right ring and the atom attached to it.
Although there are additional resonance structures to consider, it is not straightforward to determine which ring gains more positive charge by resonance alone. Some resonance forms that are commonly omitted, such as those with positive charges located on oxygen, also influence the net charge distribution and complicate a simple explanation.
Challenges and Computational Considerations
Predicting the exact location of positive charge on the phenyl rings is difficult. Factors like orbital coefficients in the Lowest Unoccupied Molecular Orbital (LUMO) or the molecule’s net dipole moment offer clues but may provide conflicting answers. Expert opinions often suggest that computational chemistry methods are necessary to determine the precise charge distribution accurately.
Therefore, relying solely on resonance or qualitative models might not yield a definitive conclusion regarding the extent of positive charge on either ring.
Practical Implications
From a practical standpoint, knowing which phenyl ring bears more positive charge in such systems is rarely crucial. Most chemical reactivity and property discussions depend on broader electronic environments rather than such subtle differences. Often, the question holds more theoretical than applied value.
Summary of Key Points
- The right phenyl ring generally holds more positive charge due to resonance involving oxygen.
- Electron donation by oxygen creates a negative resonance form, partially canceling positive charge on that ring.
- Predicting charge localization is complex and may require computational analysis for accuracy.
- Omitted resonance structures, especially with positive charges on oxygen, influence net charge distribution.
- In practice, the precise distribution of positive charge between rings is often not critical.
Which phenyl ring carries more positive charge?
The right phenyl ring tends to bear more positive charge. The oxygen donates electrons that offset positive charge, making the left ring nearly neutral.
Can resonance structures clearly show where the positive charge is localized?
Not really. Resonance shows potential distributions, but predicting exact positive charge location on rings is difficult and sometimes unclear.
Is there a simple rule to decide which ring holds more positive charge?
No straightforward rule exists. Computational methods or luck are often needed to determine which ring bears more positive charge reliably.
Does knowing which ring is more positive have practical application?
This question often lacks practical use. The distribution is complex, and focusing on it rarely impacts real-world outcomes.
Why is considering omitted resonance structures important?
Some overlooked resonance forms, like those with a positive charge on oxygen, affect charge distribution. Ignoring these may lead to incomplete conclusions.




Leave a Comment