SN1, SN2, E1, E2 Tips and Tricks: A Practical Guide for Reaction Prediction

Distinguishing between SN1, SN2, E1, and E2 reactions depends primarily on substrate type, nucleophile/base strength, solvent, and temperature. By understanding these factors, one can efficiently predict which reaction pathway will dominate under given conditions.
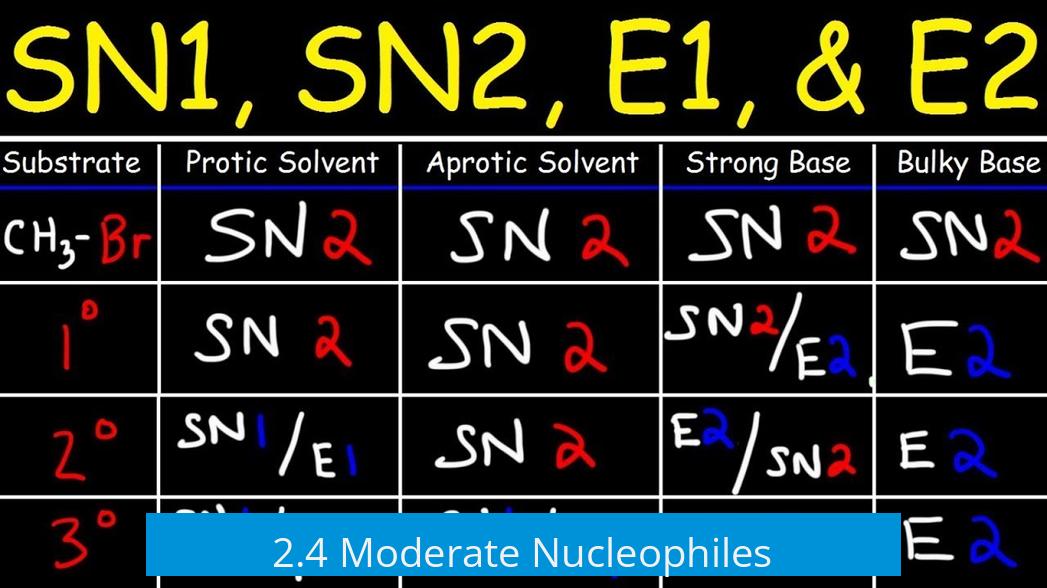
1. Organizing Predictions with a Reaction Chart
Creating a chart with alkyl halide types on one axis and nucleophile/base types on the other simplifies reaction prediction. This visual aids memorization and clarifies which mechanism dominates for each combination.
- Substrate classes: methyl, primary (1°), secondary (2°), tertiary (3°)
- Nucleophile/base strength: weak, moderate, strong
- Fill chart cells with expected mechanisms: SN1, SN2, E1, or E2
This approach provides a quick lookup method that supports logical reasoning rather than guesswork.
2. Rules Based on Substrate and Nucleophile/Base Strength
2.1 Methyl and Primary Halides

- Methyl halides undergo SN2 exclusively due to lack of steric hindrance.
- Primary halides generally favor SN2 regardless of nucleophile strength, except when bulky or strong bases (like alcoholic KOH, sodamide) induce E2.
2.2 Secondary and Tertiary Halides with Weak Nucleophiles
Weak nucleophiles (neutral molecules such as alcohol or water) cannot support SN2 or E2 on hindered substrates. This leads to SN1 or E1 reactions.
- At temperatures below 50°C, SN1 dominates due to carbocation formation.
- Above 50°C, elimination (E1) is preferred.
2.3 Strong Nucleophiles and Bases
Strong nucleophiles/bases like alcoholic KOH, alcoholic methoxide, and sodamide promote SN2 and E2 across all alkyl halides.
- These reagents favor E2 elimination when available, even for tertiary alkyl halides.
- Strong nucleophiles do not rely on carbocation formation, proceeding via concerted mechanisms.
2.4 Moderate Nucleophiles
Charged nucleophiles such as halide or acetate offer mixed behaviors:
- Secondary substrates: favor SN2 substitution.
- Tertiary substrates: proceed via SN1 or E1, especially when heated.
- Heat increases elimination tendencies.
3. Notable Exceptions and Special Cases
- Tert-butoxide almost exclusively leads to E2 elimination due to its strong, bulky base nature.
- Alkyl fluorides undergo E1cB elimination, a conjugate base elimination route favored by poor leaving groups.
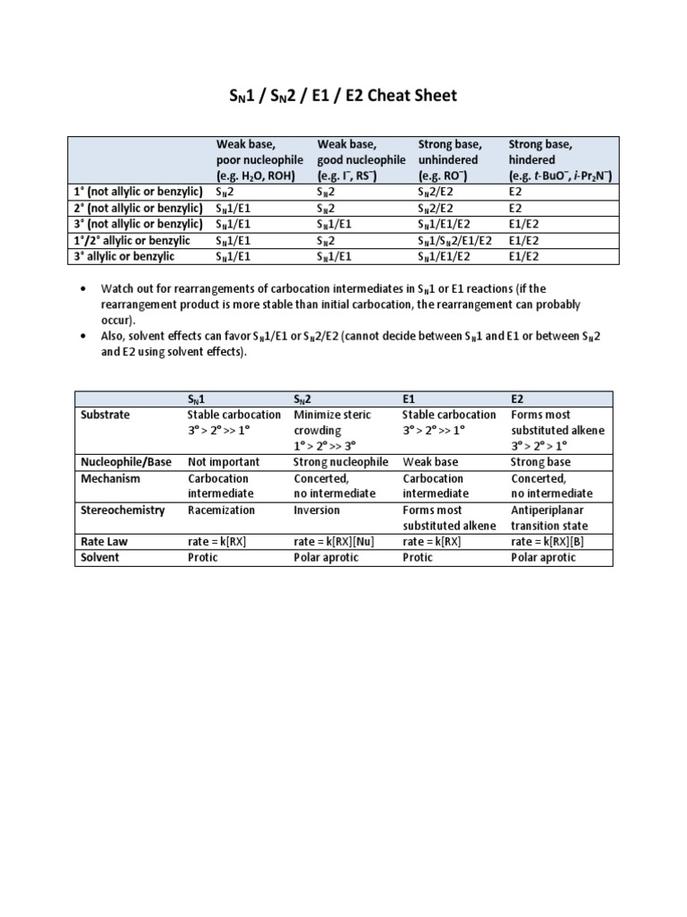
4. Four Key Criteria to Decide Mechanism
| Criterion | Effect on Mechanism |
|---|---|
| Nucleophile/Base Strength | Strong basic nucleophile: SN2/E2; strong non-basic nucleophile: SN2; strong non-nucleophilic base: E2/E1cB; weak base/nucleophile: SN1/E1 |
| Solvent Type | Polar protic favors SN1/E1; polar aprotic favors SN2/E2 |
| Substrate Substitution | More substituted favors SN1/E1; allylic/benzylic enables all; carbonyl presence favors SN2/E2; beta-carbonyl favors E1cB |
| Leaving Group Quality | Good leaving group facilitates all; poor leaving groups favor E1cB |
5. Thought Process to Predict Reaction Mechanism
- List reactants to assess nucleophile strength and leaving group quality.
- Determine if carbocation formation is possible – indicates SN1/E1.
- Analyze if elimination pathways can occur, considering base strength and substrate.
- Match substitution pattern: tertiary substrates lean toward SN1/E1; primary and secondary lean toward SN2/E2.
This stepwise reasoning aids in avoiding guesswork and ensures efficient prediction.
6. Summary Table of Trends
| Substrate Type | Weak Nucleophile/Base | Moderate Nucleophile/Base | Strong Base/Nucleophile |
|---|---|---|---|
| Methyl | SN2 only | SN2 | SN2/E2 |
| Primary (1°) | Mostly SN2 | SN2 | SN2/E2 (except with bulky bases like alc KOH) |
| Secondary (2°) | SN1/E1 (heat-dependent) | SN2 | SN2/E2 |
| Tertiary (3°) | SN1/E1 (E1 favored at high temp) | SN1/E1 | E2 dominant |
7. Additional Tips
- Elevated temperatures favor elimination (E1 or E2) over substitution.
- Polar protic solvents stabilize carbocations, promoting SN1/E1.
- Bulky bases steer reactions toward elimination rather than substitution.
- Strong nucleophiles tend to favor bimolecular mechanisms (SN2, E2).
8. Recommended Resources for Further Study
- Master Organic Chemistry: Substrate Perspective
- OpenStax Organic Chemistry Textbook – Chapter 10
- Organic Chemistry Tutoring Canada: Reaction Tables
Key Takeaways
- Use a substrate vs. nucleophile/base chart to visualize reaction types.
- Predict SN2 for methyl/primary halides with strong nucleophiles.
- Tertiary halides with weak nucleophiles favor SN1/E1; heat enhances elimination.
- Strong nucleophiles and bases promote E2, even on tertiary substrates.
- Bulky bases such as tert-butoxide exclusively cause E2.
- Consider solvent polarity, leaving group, and carbocation formation in decision.
- Practice logical analysis rather than memorization for reliable predictions.
Mastering SN1, SN2, E1, and E2: Tips and Tricks to Predict Organic Reactions Like a Pro
Wondering which reaction mechanism your alkyl halide will follow? The answer lies in understanding your substrate type, nucleophile/base strength, solvent, and temperature. It sounds like a lot, but with a little strategy, you can predict SN1, SN2, E1, and E2 like a seasoned chemist. Ready to crack the code? Let’s break it down!
If you’ve ever stared blankly at a reaction mechanism and asked, “Is it substitution or elimination? SN1 or SN2?” — you’re not alone. Thankfully, there’s a tried-and-true strategy that can bring clarity: starting with a simple chart.
Getting Organized: The Reaction Prediction Chart

Drawing a chart with alkyl halide types on one axis and nucleophile/base types on the other helps you visualize the entire landscape. You fill in the boxes with the likely reaction type. This isn’t just a study trick; it helps memorize typical pathways and exceptions at a glance.
| Substrate Type | Weak Nucleophile/Base | Moderate Nucleophile/Base | Strong Base/Nucleophile |
|---|---|---|---|
| Methyl | SN2 only | SN2 | SN2/E2 |
| Primary (1°) | SN2 mostly | SN2 | SN2/E2 (except with alc KOH, alc ethanol, sodamide) |
| Secondary (2°) | SN1/E1 (temperature-dependent) | SN2 | SN2/E2 |
| Tertiary (3°) | SN1/E1 (heat favors E1) | SN1/E1 | E2 dominant |
With this chart, deciding *which* reaction is more likely turns from guesswork into pattern recognition.
Substrate and Nucleophile Strength: Your First Clues
Let’s start at the simplest end — methyl halides: SN2, always. No exceptions here. Why? Because no carbocation can form, so the reaction needs a strong nucleophile directly attacking.
Primary halides mostly follow the same ballgame and favor SN2. The only twist is if you have special reagents like alcoholic KOH, alcoholic ethanol, or sodamide—they push the reaction to E2.
Secondary and tertiary halides shake things up. Weak nucleophiles—think water or alcohol—can’t force a direct attack or strong elimination. Instead, the molecule undergoes SN1 or E1. Here’s the kicker: Temperature sets the tone. Above 50°C? It’s elimination (E1) all the way.
Strong Nucleophiles: The Reaction Dictators
Strong nucleophiles such as alcoholic KOH, methoxide, or sodamide are the bossy kids in school—they dictate E2 or SN2 regardless of the substrate. From primary to tertiary, these reagents promote E2, sometimes backed by SN2 when the substrate allows.
Moderate Nucleophiles in the Mix

Moderate nucleophiles—charged but not overly aggressive, like halides or acetate—play a nuanced game. For example, they favor SN2 when the substrate is secondary but tend to back off to SN1 or E1 on tertiary. Again, heat is the secret ingredient pushing elimination over substitution.
Special Cases You Shouldn’t Miss
- tert-Butoxide: This bulky base is an E2-only machine. Seriously, no exceptions; steric hindrance blocks substitution.
- Alkyl fluorides: Not your average elimination—these pass through an E1CB (E1 conjugate base elimination) pathway.
It’s like having special weapons in your organic chemistry arsenal—know them, respect them.
Solvent Effects: The Unsung Hero
Don’t forget solvents. Polar protic solvents—think water, alcohols—favor SN1 and E1 reactions by stabilizing carbocations. On the flip side, polar aprotic solvents like DMSO or acetone favor SN2 and E2 because they don’t stabilize ions as much, allowing nucleophiles to stay reactive.
Put it All Together: Your Thought Process
Picture this: You’re handed a reaction. Where do you start?
- List your reactants: identify nucleophile strength and the leaving group’s quality.
- Ask yourself: can a carbocation form? If yes, SN1 is likely.
- Look for elimination chances: strong bases and substrate structure may push E2 or E1 pathways.
- Check your substrate: tertiary favors SN1/E1; primary and secondary lean toward SN2/E2.
It sounds like a lot, but writing everything down sharpens focus. The amazing thing? Once you get this down, predicting reactions feels less like guesswork and more like detective work.
Why Does This Matter? The Benefits of Mastering These Tips
Predicting reaction pathways isn’t just academic—it improves lab efficiency and prevents costly mistakes. Knowing whether elimination or substitution will dominate helps in synthesis planning. For example, if you want to install a nucleophile, you avoid conditions that favor elimination.
Plus, thinking logically reduces panic during exams. Instead of memorizing endless pathways, you understand *why* reactions behave a certain way—and that’s powerful.
Real-Life Example: Applying These Rules
Let’s say you have 2-bromopropane (a secondary halide) and sodium methoxide in methanol. What happens?
- Substrate is secondary.
- Nucleophile is strong (methoxide).
- Solvent is polar protic (methanol).
Conclusion: Both SN2 and E2 are possible. But since methoxide is a strong base/nucleophile and substrate is secondary, E2 often wins especially at higher temperatures. Cool, huh?
Final Summary: Your Cheat Sheet
- Draw a reaction prediction chart with substrate and nucleophile/base types.
- Memorize key rules: methyl & primary → mostly SN2; tertiary + weak nucleophiles → SN1/E1; strong bases → E2 dominant.
- Always factor temperature: Above 50°C means elimination (E1) for weak nucleophiles.
- Remember solvent type impacts the mechanism.
- Know your exceptions: tert-butoxide for E2 only; alkyl fluorides for E1CB.
- Use a logical thought process to analyze your reactants.
By combining these approaches—you’ll feel confident predicting SN1, SN2, E1, and E2 reactions faster and more accurately. Plus, you get to flex your chemistry brain like a true pro!
Need More Practice?
Check out these excellent resources to deepen your understanding:
- Master Organic Chemistry’s SN1/SN2/E1/E2 guide – great explanations on substrate influence
- OpenStax Organic Chemistry – concise textbook with full mechanisms
- Organic Chemistry Tutoring Canada reaction tables – handy summary charts
And remember: mistakes are part of the learning. Each wrong prediction is one step closer to mastery. So grab your lab coat (or study guide), and start applying these tips today!
Q1: How can a chart help in predicting SN1, SN2, E1, and E2 reactions?
Drawing a chart with alkyl halide types on one axis and nucleophile/base types on the other helps organize which mechanism predominates. Filling in reaction types for each combination improves memory and generalization.
Q2: When do strong nucleophiles favor E2 over SN2?
Strong nucleophiles like alc KOH or sodamide usually cause E2 to dominate, even with primary or secondary halides. They promote elimination because they are strong bases designed for this.
Q3: Why does temperature affect whether E1 or SN1 happens with tertiary halides?
At temperatures above 50°C, elimination (E1) predominates for tertiary halides with weak nucleophiles. Below that, SN1 and E1 compete, but heat shifts the pathway toward elimination.
Q4: What role does solvent play in choosing between these mechanisms?
Polar protic solvents favor SN1 and E1 due to stabilization of carbocations. Polar aprotic solvents favor SN2 and E2 by enhancing nucleophile strength and base action without stabilizing carbocations.
Q5: How can I decide between SN1, SN2, E1, and E2 using substrate and reagent info?
- Check nucleophile/base strength: strong bases favor SN2/E2; weak favor SN1/E1.
- Assess substrate: tertiary favors SN1/E1; primary and methyl favor SN2.
- Consider temperature: higher favors E1.
- Look for good leaving groups and solvent type to fine-tune prediction.



Leave a Comment