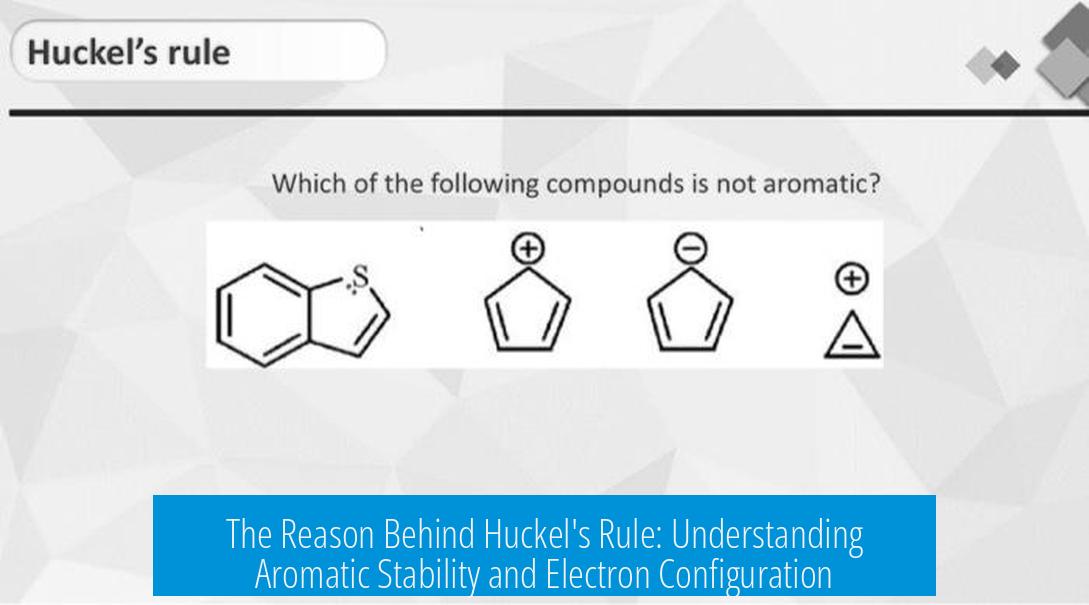
The Reason Behind Huckel’s Rule

Hückel’s rule states that planar, cyclic, fully conjugated molecules exhibit aromatic stability when they contain 4n+2 π electrons (where n is an integer). This rule arises from the molecular orbital (MO) configuration of such systems. Specifically, the rule reflects electron filling patterns in molecular orbitals shaped as 2D standing waves. The key to stability lies in fully paired electrons occupying energy orbitals without unpaired electrons or partially filled orbitals, which only happens when the π electron count equals 4n+2.
Molecular Orbital Configuration in Planar Conjugated Cyclic Systems
In fully conjugated planar cyclic molecules, the π molecular orbitals arrange systematically. The lowest energy level contains a single MO without nodes (except in-plane nodes), followed by pairs of degenerate orbitals at increasing energy levels. This pattern repeats, forming sets of energy levels with two orbitals each, except the lowest orbital which stands alone.
- Lowest energy level: 1 orbital (no degenerate partner)
- Next levels: pairs of orbitals, degenerate in energy
This arrangement is a direct consequence of the symmetry and boundary conditions within the cyclic π system. The pattern of nodes (regions where the wavefunction changes sign) defines these energy levels.
Standing Wave Model of Aromatic Orbitals
Each molecular orbital in the cyclic system behaves like a two-dimensional standing wave arranged around a ring. Nodes are lines where the wavefunction passes through zero. Apart from the lowest and highest energy orbitals, each level has two possible orientations of these nodal lines—commonly at right angles to each other.
- The lowest energy orbital has no nodes inside the ring (bonding orbital).
- Each higher energy level contains two degenerate orbitals, differing by the orientation of nodes.
- The highest energy orbital normally has nodes at every atom and is antibonding.
This wave nature dictates electron occupancy and orbital energies.
Electron Filling and Aromatic Stability
Electrons fill the molecular orbitals starting from the lowest energy.
- The lowest orbital fits 2 electrons.
- Each pair of degenerate orbitals accommodates up to 4 electrons (2 electrons per orbital).
For maximum stability, all occupied orbitals must be fully paired. Partially filled or singly occupied orbitals introduce instability.
This requirement for fully paired orbitals leads to the electron count rule:
| Energy Level | Number of Orbitals | Electrons per Level | Total Electrons (Sum) |
|---|---|---|---|
| Lowest | 1 | 2 | 2 |
| Next n levels (pairs) | 2 per level | 4 per level | +4n |
| Total | 2 + 4n |
This total, 4n+2 π electrons, corresponds exactly to stable aromatic molecules, as dictated by Hückel’s rule.
Instability of 4n Electron Systems: Antiaromaticity
Molecules with 4n π electrons tend to be destabilized in a planar aromatic geometry. Their molecular orbitals lead to unpaired electrons (diradicals) or electrons occupying non-bonding or antibonding orbitals.
- Diradicals increase reactivity and lower stability.
- Partial occupancy means electrons reside in higher energy or destabilizing orbitals.
- These molecules often distort out of planarity to avoid antiaromatic destabilization.
This explains the absence of aromatic stabilization for 4n electron systems. Such molecules are called antiaromatic when they maintain planarity and conjugation.
Graphical Visualization: Frost Circles and Orbital Diagrams
A useful tool to visualize the MO energies in cyclic conjugated systems is the Frost circle construction.
- Draw a regular polygon inside a circle with one vertex downward.
- Energy levels correspond to the vertices on the circle’s circumference.
- Electrons fill MOs from the lowest point upwards.
Frost circles clearly show that with 4n+2 electrons, all orbitals below the highest occupied molecular orbital (HOMO) are fully filled. With 4n electrons, the HOMO becomes half-filled or an electron populates an antibonding orbital, explaining the instability.
Summary of the Key Physical Basis
- Planar cyclic conjugated molecules have molecular orbitals shaped as standing waves with defined nodal patterns.
- Electron filling follows the pattern of 1 orbital at lowest energy and pairs of degenerate orbitals at higher levels.
- Only π electron counts of 4n+2 result in fully paired orbital occupancy without partially filled or unpaired electron shells.
- Systems with 4n π electrons experience destabilization due to unpaired electrons or electrons entering antibonding orbitals.
- This leads to the aromatic stability of 4n+2 electron systems and antiaromatic instability of 4n systems.
Remaining Fundamentals and Philosophical Notes
Despite these models and visualizations, some consider the “why” behind the stability of 4n+2 electrons more philosophical than fully explained. Molecular orbital theory predicts the patterns well, but the absolute fundamental cause for the specific rule sits at the intersection of quantum mechanics, symmetry, and electron interactions.
More complex theoretical and computational studies continue to investigate the deeper origins of aromatic stability patterns.
Additional Resources for Visualization and Learning
To understand Hückel’s rule more intuitively, exploring molecular orbital models graphically helps greatly:
- Benzene Molecular Orbitals — Visualization of bonding and antibonding orbitals.
- 2D Standing Waves on a Circle — Interactive demonstrations of nodal patterns.
- Drawing Frost circles for rings of various sizes (4 to 8 atoms) clarifies electron filling patterns for aromatic and antiaromatic systems.
Key Takeaways
- Hückel’s rule arises from the molecular orbital structure of planar cyclic conjugated molecules.
- The stable filling pattern requires 4n+2 π electrons to avoid partially filled or unpaired orbitals.
- Molecular orbitals correspond to 2D standing waves with discrete nodal patterns dictating energies.
- Systems with 4n electrons populate antibonding or non-bonding molecular orbitals, causing instability.
- Graphical tools like Frost circles aid in visualizing orbital energies and electron occupancy.
- The fundamental root of the rule ties into quantum mechanics but retains some open conceptual questions.
Why does Huckel’s rule require 4n+2 π electrons for aromaticity?
Huckel’s rule arises because the lowest molecular orbital holds 2 electrons, and each higher degenerate pair holds 4. To avoid partially filled orbitals, stable systems have 2 plus multiples of 4 electrons, making 4n+2.
What causes systems with 4n π electrons to be unstable or antiaromatic?
Systems with 4n π electrons have unpaired electrons or electrons in non-bonding orbitals. This creates diradical character, destabilizes the molecule, and prevents a planar structure needed for aromatic stabilization.
How do molecular orbitals behave in planar cyclic conjugated molecules?
The orbitals form 2D standing waves with nodes. Except for the lowest and highest energy orbitals, each energy level has two degenerate orbitals with different nodal orientations, defining the electron distribution and energy.
Why is the exact fundamental reason for Huckel’s rule still unclear?
Although molecular orbital theory predicts stability patterns, the deeper, fundamental “why” behind the 4n+2 electron count remains unresolved. It borders on a philosophical question beyond current models’ scope.
How can Frost circles help understand Huckel’s rule?
Frost circles graphically show molecular orbitals for cyclic systems. By placing 4n or 4n+2 electrons on these diagrams, you can see which orbitals fill completely, revealing why certain counts lead to aromatic or antiaromatic behavior.



Leave a Comment