Useful Named Reactions in Organic Chemistry
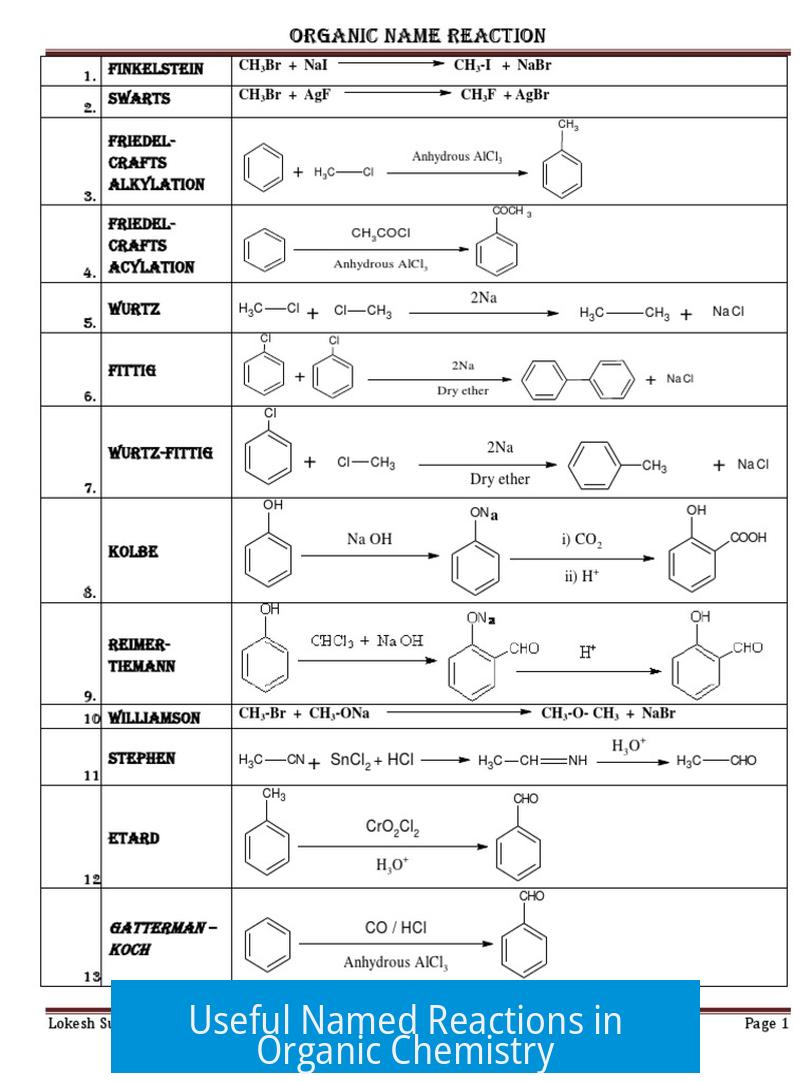
Named reactions in organic chemistry provide essential tools for synthesizing complex molecules efficiently and selectively. These reactions are fundamental in research, pharmaceuticals, and industrial applications due to their reliability and versatility.
Understanding Named Reactions

Named reactions are specific chemical transformations named after their discoverers or developers. These reactions often have well-established mechanisms and broad applications in synthetic organic chemistry. Learning them equips chemists with a toolkit for constructing molecules with desired structures and functions.
Why Learn Named Reactions?
- Facilitate retrosynthetic analysis.
- Enable efficient assembly of molecular frameworks.
- Are widely used in medicinal chemistry and industry.
- Help in understanding reaction mechanisms and selectivity.
For example, in medicinal chemistry, the Suzuki coupling is indispensable for forming carbon-carbon bonds. It is considered a must-know reaction for synthetic chemists due to its versatility and mild conditions.
Classification by Reaction Type
Organizing named reactions by their types helps in learning and application:
| Reaction Type | Example Named Reactions | Applications |
|---|---|---|
| Coupling Reactions | Suzuki, Heck, Stille, Buchwald-Hartwig, Chan-Lam | Forming C–C and C–N bonds in drug synthesis |
| Oxidation Reactions | Dess-Martin, Swern, Jones, Baeyer-Villiger | Introducing or modifying functional groups |
| Reduction Reactions | Birch reduction, Wolff-Kishner | Converting functional groups to less oxidized forms |
| Addition and Condensation | Michael addition, Aldol addition/condensation, Claisen condensation | Building carbon skeletons via bond formation |
| Cycloaddition Reactions | Diels-Alder, Huisgen dipolar cycloaddition | Constructing ring systems |
| Rearrangement Reactions | Claisen rearrangement | Structural reorganization of molecules |
| Functional Group Interconversion | Wittig reaction, Mitsunobu reaction, Williamson ether synthesis | Transforming or creating new functional groups |
Key Named Reactions and Their Significance
Coupling Reactions
- Suzuki Coupling: Palladium-catalyzed cross-coupling between organoboron compounds and halides. Extremely important in medicinal chemistry for biaryl synthesis.
- Heck Reaction: Palladium-catalyzed coupling of alkenes with aryl halides to form substituted alkenes.
- Stille Coupling: Cross-coupling of organostannanes with halides.
- Buchwald-Hartwig Amination: Palladium-catalyzed coupling to form C–N bonds between aryl halides and amines.
- Chan-Lam Coupling: Copper-mediated coupling of arylboronic acids with amines or alcohols at mild conditions.
Oxidation and Reduction
- Dess-Martin Oxidation: Converts primary and secondary alcohols to aldehydes and ketones using Dess-Martin periodinane.
- Swern Oxidation: Mild oxidation of alcohols to aldehydes or ketones utilizing DMSO and oxalyl chloride.
- Jones Oxidation: Strong oxidation with chromium reagents, effective for converting alcohols to carboxylic acids.
- Baeyer-Villiger Oxidation: Converts ketones to esters or lactones using peracids.
- Birch Reduction: A dissolving metal reduction of aromatic rings producing dihydroaromatics.
- Wolff-Kishner Reduction: Converts ketones and aldehydes to alkanes under strongly basic conditions.
Addition, Condensation, and Rearrangement
- Aldol Addition and Condensation: Form carbon-carbon bonds via enolate reaction with aldehydes or ketones.
- Michael Addition: 1,4-conjugate addition to α,β-unsaturated carbonyl compounds.
- Claisen and Dieckmann Condensations: Carbon–carbon bond-forming reactions involving esters.
- Claisen Rearrangement: Sigmatropic rearrangement involving allyl vinyl ethers to form γ,δ-unsaturated carbonyl compounds.
Cycloaddition and Special Reactions
- Diels-Alder Reaction: [4+2] cycloaddition between dienes and dienophiles forming six-membered rings.
- Huisgen Dipolar Cycloaddition: Often called “click chemistry,” a [3+2] cycloaddition between azides and alkynes useful for bioconjugation.
- Mitsunobu Reaction: Inverts alcohol stereochemistry via nucleophilic substitution.
- Williamson Ether Synthesis: An SN2 reaction that constructs ethers from alkoxides and alkyl halides.
Additional Noteworthy Reactions
The Vilsmeyer-Haack Reaction uses DMF and POCl3 to formylate aromatic and heteroaromatic rings. It is particularly valued in synthesizing pyrazole derivatives and understanding electrophilic aromatic substitution mechanisms.
The Formose Reaction is an autocatalytic process converting formaldehyde into sugars. It remains biologically relevant, providing insights into prebiotic chemistry.
The Cannizzaro Reaction is a disproportionation of non-enolizable aldehydes to alcohols and carboxylic acids in basic media. Despite lacking broad fame, it plays a niche role in synthesis.
The Staudinger Reaction allows azides to be converted to amines through phosphine intermediates. This reaction and its derivatives have important biochemical applications, including biomolecule labeling essential for molecular biology.
Named vs. Unnamed Reactions
Not all frequently used organic transformations are named. Mechanistically, some named and unnamed reactions resemble each other closely. For example, the Claisen condensation is named after the chemist Claisen, whereas the similar Aldol condensation is generally not named. The classification often depends on historical factors and the uniqueness of the reaction’s discovery or application.
Industrial and Pharmaceutical Relevance
Industry heavily relies on certain named reactions due to their reliability and scalability. The Suzuki coupling dominates in pharmaceutical syntheses of complex molecules. Likewise, Buchwald-Hartwig amination outperforms Ullmann coupling for forming C–N bonds under milder conditions with higher yields.
Online communities such as r/ChemicalEngineering provide insight into industrially relevant processes and the practical popularity of reactions.
Recommended Resources for Further Learning
- Strategic Applications of Named Reactions in Organic Synthesis: A comprehensive textbook resource.
- Pharma-Relevant Reactions Paper: Discusses commonly used pharmaceutical reactions, both named and unnamed.
Summary of Key Named Reactions
| Named Reaction | Main Application | Reaction Type |
|---|---|---|
| Suzuki Coupling | Formation of biaryl compounds | C-C Coupling |
| Buchwald-Hartwig Amination | C-N bond formation | C-N Coupling |
| Diels-Alder Reaction | Ring synthesis via cycloaddition | Cycloaddition |
| Dess-Martin Oxidation | Alcohol to carbonyl oxidation | Oxidation |
| Michael Addition | 1,4-Conjugate addition | Addition |
| Claisen Condensation | Carbon–carbon bond formation | Condensation |
| Huisgen Cycloaddition | Bioconjugation (click chemistry) | Cycloaddition |
Key Takeaways
- Named reactions are fundamental for constructing complex organic molecules.
- The Suzuki coupling is a cornerstone of medicinal chemistry.
- C-N coupling reactions like Buchwald-Hartwig and Chan-Lam complement C-C couplings.
- Grouping reactions by type aids comprehension and practical use.
- Many named reactions overlap mechanistically with unnamed counterparts.
- Resources like “Strategic Applications of Named Reactions” help deepen understanding.
- Industrial relevance guides selection of essential reactions for study.
What are some key named reactions every organic chemistry student should know?
Students should focus on Suzuki coupling, Buchwald-Hartwig amination, and Chan-Lam couplings due to their frequent use in medicinal chemistry.
Also, reactions like Diels-Alder, Wittig, and Aldol additions are fundamental.
Why might grouping named reactions by type be useful?
Grouping by type, like oxidations, reductions, or couplings, helps in understanding mechanisms and industrial application.
It also aids memorization and connects related reaction pathways.
How is the Vilsmeyer-Haack reaction unique or important?
It uses DMF and POCl3 to formylate aromatics, useful in making heterocycles like pyrazole rings.
The mechanism demonstrates versatile uses of common reagents.
What role do named reactions play in biochemistry and molecular biology?
Reactions like the Staudinger reaction allow for labeling and tagging biomolecules, important in bio-related labs.
Huisgen dipolar cycloaddition also has valuable bio-applications.
How do named reactions compare to unnamed ones?
Many named reactions share mechanisms with unnamed ones, like Claisen versus Aldol condensation.
Often, naming recognizes historical discovery rather than distinct chemistry differences.


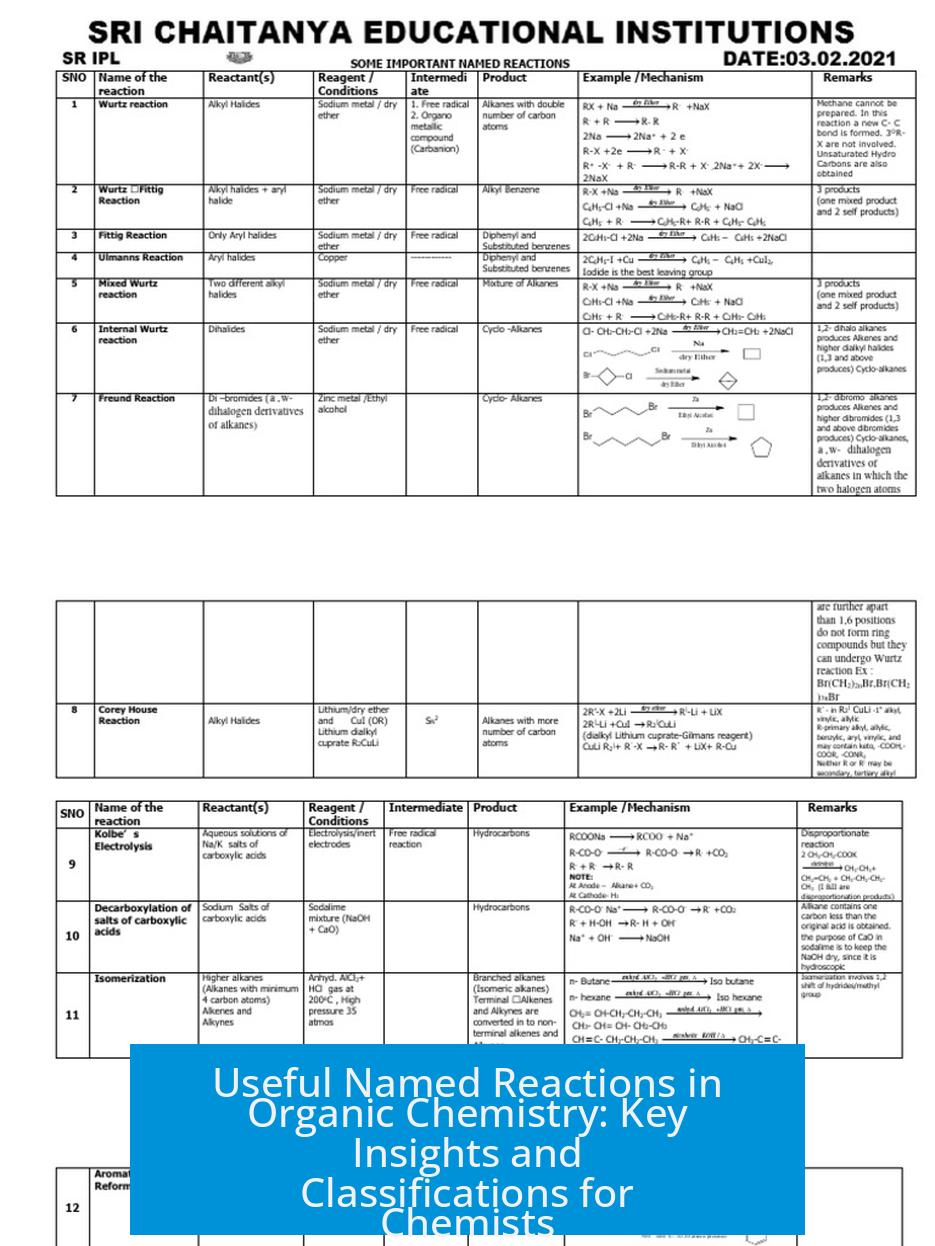


Leave a Comment