Atoms are the building blocks of life, and understanding how they work can help us to better comprehend the universe around us. The number of protons, neutrons, and electrons found in an atom can help determine its properties and how it interacts with other elements. In this article, we’ll explore what elements have 17 protons, 18 neutrons, and 18 electrons and the importance of this information.
Atoms are made up of protons, neutrons, and electrons, which are held together by electromagnetic forces. The number of protons in an atom is known as its atomic number, and this is what determines an element’s position in the Periodic Table. Normally, there is approximately the same number of protons, neutrons, and electrons in an atom, resulting in a net neutral charge and a stable nucleus.
So, what element has 17 protons, 18 neutrons, and 18 electrons? The answer is Chlorine (Cl), which has an atomic number of 17. Chlorine belongs to the Halogen group of elements and has a wide range of uses, such as being a component of household cleaning products, a bleaching agent, and a disinfectant.
Chlorine is one of the many metals that can be found in metallic meteorites, as well as in the dense metal cores of planets such as Earth. It is also an important component of stainless steel, which is an alloy of steel, chromium, and manganese. Stainless steel is a very strong and durable material, with a yield strength of 1,420 Mpa and a tensile strength of 1,460 Mpa.
In conclusion, the element that has 17 protons, 18 neutrons, and 18 electrons is Chlorine (Cl). Chlorine has a wide range of uses, from household cleaning products to stainless steel. Understanding the properties of chlorine and other elements is key to understanding the structure of the universe.
What element has 17 protons 18 neutrons and 18 electrons charge?
Atoms are composed of protons, neutrons, and electrons. These three particles have different charges and masses. Protons have a positive charge, neutrons have no charge, and electrons have a negative charge. Normally, there is approximately the same number of protons, neutrons and electrons in an atom, this results in a net neutral charge and a stable nucleus.
Question: A chlorine atom has 17 protons, 18 neutrons, and 17 electrons. What is the atomic number of chlorine?
The answer is B) 17. This is because the atomic number equals the number of protons in the nucleus of an atom. Since the question states that there are 17 protons, the atomic number must be 17. The number of protons and electrons are equal in a neutral atom, so the number of electrons must also be 17. The number of neutrons can be calculated by subtracting the number of protons (17) from the mass number (35). The mass number is equal to the number of protons plus the number of neutrons, so the number of neutrons must be 18.
Atomic Number
The atomic number of an element determines its position in the Periodic Table. It is one of the most important properties of an element and atoms. Atomic numbers can help determine the mass number or number of neutrons in an atom as well. The atomic number of an element is the same as the number of protons. For example, the atomic number of chlorine is 17, which means that it has 17 protons.
Applications
The information about protons, neutrons, and electrons can be used in various applications. For example, the knowledge of the atomic number of an element can help identify the element and its properties. It can also be used to determine the stability of an atom depending on the number of protons, neutrons, and electrons.
Alloys
Alloys are combinations of metals that result in improved strength and other properties. For example, steel-iron-nickel alloy is made by combining carbon steel with nickel. This increases the yield and tensile strength of the alloy to far above those of plain old carbon steel. It has a yield strength of 1,420 Mpa and a tensile strength of 1,460 Mpa. Iron and nickel are the most abundant metals in metallic meteorites and in the dense metal cores of planets such as Earth.
Another example is stainless steel, which is a special alloy of steel, chromium, and manganese. This alloy is corrosion-resistant and is used in a wide range of applications. It is used in the construction of buildings, ships, and many other structures. It is also used in medical equipment, kitchenware, and other tools.
In conclusion, protons, neutrons, and electrons are the three major particles that make up an atom. The atomic number of an element is equal to the number of protons in its nucleus. This information can be used to identify elements and their properties, as well as to determine the stability of an atom. It can also be used to create alloys with improved strength and other properties.
What ion has 17 protons 18 neutrons 18 electrons?
Atoms are the building blocks of matter and are made up of three subatomic particles: protons, neutrons, and electrons. The number of protons determines the element, while the number of neutrons and electrons determines the isotope and charge of the atom. When the number of protons and electrons are equal, then the element exist in pure form, otherwise, it exists as an ion.
Answering the Question
We are given an element which has 17 protons and 18 electrons. There is an extra electron present in the element as compared to protons. Hence, the atom exists as an ion with a -1 charge. The element is Chlorine and its ion is Chloride. The symbol for the ion is Cl-.
Atoms and Ions
Atoms are the basic building blocks of matter, and they consist of protons, neutrons, and electrons. Protons have a positive charge, neutrons have no charge, and electrons have a negative charge. The number of protons in an atom determines its element.
When an atom has an equal number of protons and electrons, it is in its neutral form. When the number of protons and electrons are not equal, however, the atom is an ion. An ion has either a positive or negative charge, depending on whether there are more protons or electrons.
Properties of Chlorine
Chlorine is a chemical element with an atomic number of 17, which means it has 17 protons in its nucleus. It is a halogen, which means it is a non-metal that is highly reactive. Chlorine is a very common element, found in seawater, and is used in many industrial processes, including the production of plastics, pharmaceuticals, and pesticides.
Chloride Ion
The chloride ion is the anion of chlorine, meaning that it has one more electron than protons. The chloride ion has a -1 charge and is an essential component of many biological processes in animals and plants. It is also found in large amounts in seawater and is used in the production of chlorine-based chemicals.
In conclusion, an ion from a given element with 17 protons and 18 electrons has a -1 charge and is known as a chloride ion. The element from which it originates is chlorine, and its symbol is Cl-. Chlorine is a halogen, meaning it is a non-metal that is highly reactive, and it is found in large amounts in seawater. The chloride ion is an essential component of many biological processes in plants and animals, and it is used in the production of chlorine-based chemicals.
Which element has 17 protons 22 neutrons and 17 electrons?
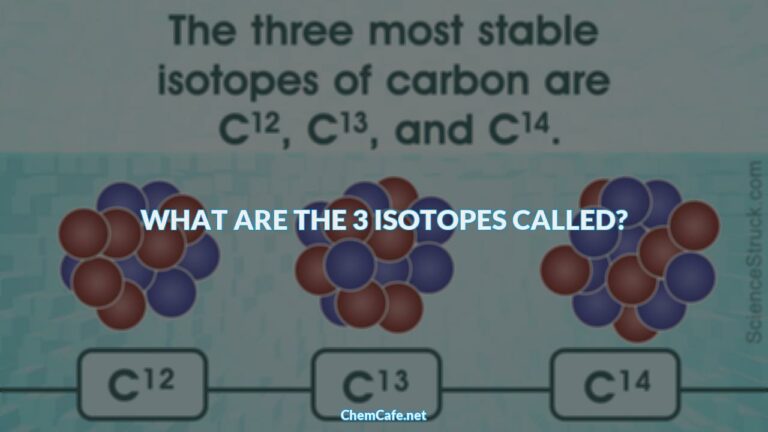
Atoms of the same element differ in the number of neutrons they contain, giving rise to isotopes of the same element. One such element is chlorine, which has 17 protons and 17 electrons. But the number of neutrons in chlorine can vary. Out of four chlorine atoms, three weigh 35 amu (17 protons and 18 neutrons) while the fourth weighs 37 amu (17 protons and 20 neutrons). These are the isotopes of chlorine, whose atomic weight is reported as the average weight of 35.5 amu.
How Many Protons and Neutrons are Present in Each of These Three Isotopes?
All magnesium isotopes have 12 protons. The isotope whose atomic weight is 23.985 amu has a mass number of 24 (protons and neutrons), so 24 – 12 protons gives 12 neutrons. The symbol for this isotope is 24Mg.
Writing Symbols for Each Isotope
Atoms of the same element can have different number of neutrons, giving rise to isotopes of the same element. One such element is chlorine, which has 17 protons and 17 electrons. But the number of neutrons in chlorine can vary. Out of four chlorine atoms, three weigh 35 amu (17 protons and 18 neutrons) while the fourth weighs 37 amu (17 protons and 20 neutrons). The symbol for these isotopes is written as 17Cl and 17Cl-35 for the lighter isotope and 17Cl-37 for the heavier isotope.
Weighted Average of Atomic Weights
The atomic weight of an element is the average weight of all its isotopes, weighted according to the natural abundance of each isotope. For example, the atomic weight of chlorine is 35.5 amu. This is the weighted average of the two isotopes of chlorine, 17Cl-35 and 17Cl-37. The lighter isotope 17Cl-35 is more abundant in nature, accounting for 75.77% of the total chlorine atoms. The heavier isotope, 17Cl-37, makes up 24.23% of the chlorine atoms in nature.
Therefore, the weighted average of the atomic weights of chlorine is calculated as follows:
Weighted Average of Atomic Weights = (17Cl-35 x 0.7577) + (17Cl-37 x 0.2423) = 35.5 amu
Isotopes are atoms of the same element that differ in their number of neutrons. This difference in neutron numbers gives rise to different atomic weights for the same element. The atomic weight of an element is the weighted average of the atomic weights of all its isotopes, according to the natural abundance of each isotope. The example of chlorine helps us understand how the atomic weight of an element is calculated, and how the number of protons and neutrons present in each isotope is determined.
What element has 17 protons 20 neutrons and 18 electrons?
Atoms are the building blocks of all matter and are composed of protons, electrons, and neutrons. Every element has a unique number of protons which determines its atomic number. For example, chlorine has an atomic number of 17, meaning it has 17 protons. But the number of neutrons in an atom of that element can vary. This variation is known as isotopes.
What are Isotopes?
Isotopes are atoms of the same element that have a different number of neutrons. For example, most chlorine atoms have 17 protons and 17 neutrons, but some have 17 protons and 18 neutrons, and others have 17 protons and 20 neutrons. The atomic weight of an element is the average mass of the different isotopes.
How Many Protons and Neutrons are in Each Isotope?
The isotope of chlorine that has 17 protons and 18 neutrons has a mass number of 35 (17 protons + 18 neutrons). This isotope can be written as 35Cl. The isotope with 17 protons and 20 neutrons has a mass number of 37 (17 protons + 20 neutrons). This isotope can be written as 37Cl.
What is the Weighted Average of the Atomic Weights?
The atomic weight of chlorine is 35.453 amu, which is the weighted average of the atomic weights of the two isotopes. The atomic weight of chlorine is calculated by adding the mass number of each isotope and dividing by the number of isotopes. In this case, it would be (35 + 37) / 2 = 36.
Example: Calculating the Mass Lost When an Atom of Carbon-12 is Formed
If an atom of carbon-12 is formed from protons, electrons, and neutrons, the mass that is lost can be calculated. Carbon-12 has 6 protons and 6 neutrons, so the mass number is 12 (6 protons + 6 neutrons). The mass that is lost can be calculated by subtracting the mass number from the mass of the atom. In this case, the mass of the atom is 12 amu, so the mass that is lost is 12 amu – 12 amu = 0 amu.
In conclusion, isotopes are atoms of the same element that have different numbers of neutrons. The atomic weight of an element is the average mass of the different isotopes. The isotope of chlorine that has 17 protons and 18 neutrons can be written as 35Cl, and the isotope with 17 protons and 20 neutrons can be written as 37Cl. The atomic weight of chlorine is 35.453 amu, which is the weighted average of the atomic weights of the two isotopes. Finally, the mass that is lost when an atom of carbon-12 is formed from protons, electrons, and neutrons can be calculated by subtracting the mass number from the mass of the atom.
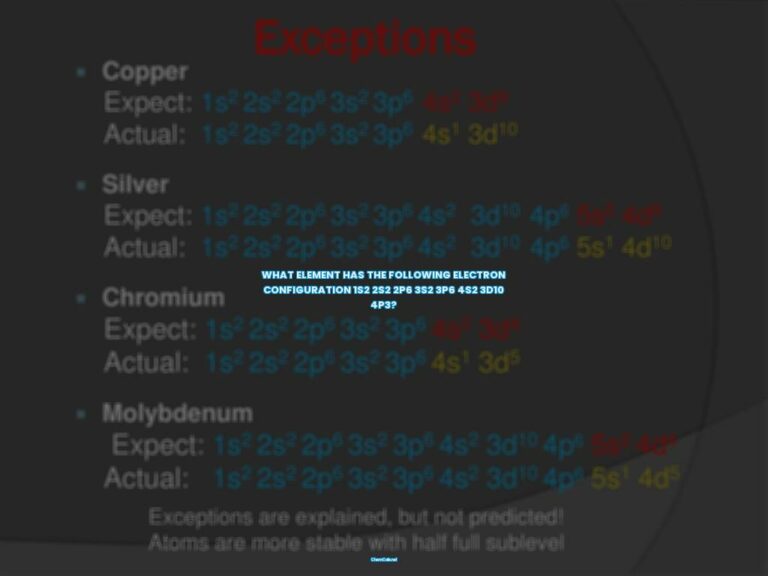
What element has 17 protons 18 neutrons and 18 electrons?
Atoms are the building blocks of matter, and each atom is made up of protons, electrons, and neutrons. The number of protons, electrons, and neutrons in an atom determines its elemental identity. For example, an atom with 17 protons, 18 neutrons, and 18 electrons is a chlorine atom.
Chlorine is a halogen, and it is the third-lightest halogen, following fluorine and bromine. It is a yellow-green gas at room temperature and pressure, and it is the 17th most abundant element in the Earth’s crust. Chlorine is an essential component of many everyday items, such as table salt and bleach.
Atomic Structure of Chlorine
Chlorine is a chemical element with an atomic number of 17, meaning it has 17 protons in its nucleus. The nucleus also contains 18 neutrons, resulting in a total mass number of 35. The electrons in the atom are arranged in shells that correspond to the number of protons. Chlorine has seven electrons arranged in three shells. The first shell contains two electrons, the second shell contains eight electrons, and the third shell contains seven electrons.
Chlorine has a complete octet in the outermost shell, which means it is stable and not easily reactive. This is why chlorine atoms do not react with other atoms unless they must in order to form a stable molecule.
Uses of Chlorine
Chlorine is an important element for many industries. It is used in the production of chlorinated solvents and other chemicals, as well as in water treatment and bleaching processes. It is also used in the manufacture of plastic, rubber, and dyes.
Chlorine is also important for the environment. It helps to keep water clean and free of bacteria and other contaminants. Chlorine is also used to remove heavy metals and other pollutants from water.
Isotopes of Chlorine
Chlorine has two stable isotopes, chlorine-35 and chlorine-37. Chlorine-35 has 17 protons, 18 neutrons, and 18 electrons, while chlorine-37 has 17 protons, 20 neutrons, and 18 electrons. The isotopes differ in their mass number, which is the sum of the protons and neutrons in the nucleus.
Chlorine also has some naturally-occurring radioactive isotopes. These isotopes have the same number of protons and electrons, but a different number of neutrons. These isotopes are unstable and have a short half-life, meaning they decay quickly.
Chlorine is an element with 17 protons, 18 neutrons, and 18 electrons. It is a yellow-green gas at room temperature and pressure and is the 17th most abundant element in the Earth’s crust. Chlorine has two stable isotopes, chlorine-35 and chlorine-37, and a few naturally-occurring radioactive isotopes. Chlorine is an important element for many industries and is also vital for keeping water clean and free of bacteria and other contaminants.
What element has 17 protons 20 neutrons and 17 electrons?
Atoms consist of three particles: protons, neutrons, and electrons. The number of protons determines the element, while the number of neutrons can vary. This variation is known as isotopes. An isotope is an atom of the same element with different numbers of neutrons.
The element with 17 protons and 20 neutrons is chlorine. Chlorine is found in the halogen group in the periodic table, and it has the atomic number 17. Chlorine atoms can have different numbers of neutrons, but they will all have 17 protons and 17 electrons.
Isotopes are atoms of the same element that have different numbers of neutrons. This can give them different atomic weights, even though they have the same number of protons and electrons. For example, most chlorine atoms have 17 protons and 17 electrons, but they can have 18 or 20 neutrons, which gives them atomic weights of 35 and 37, respectively.
The weighted average of the atomic weights of different isotopes of an element is known as the ‘atomic weight’ of that element. The atomic weight of chlorine is 35.5, which is the average of the 35 and 37 chlorine isotopes.
Isotope Notation
Isotopes are usually represented by the element’s symbol, followed by a hyphen and the mass number. For example, the chlorine isotope with 17 protons and 20 neutrons is usually written as Cl-37. The mass number is the total number of protons and neutrons in the atom.
Similarly, the isotope of magnesium with 12 neutrons is written as 24Mg. The mass number is 24, so there must be 12 protons and 12 neutrons in the atom.
The element with 17 protons, 20 neutrons, and 17 electrons is chlorine. Chlorine is a halogen with an atomic number of 17. Chlorine atoms can have different numbers of neutrons, which gives them different atomic weights. Isotopes are usually written using the element’s symbol, followed by a hyphen and the mass number. The mass number is the total number of protons and neutrons in the atom.



Leave a Comment