Isotopes are atoms of the same element that have different masses due to having different numbers of neutrons. There are over 3000 known isotopes in existence, with some being stable and others being radioactive. While different isotopes of the same element may have different properties, they still share the same atomic number, which is the number of protons in the nucleus of the atom.
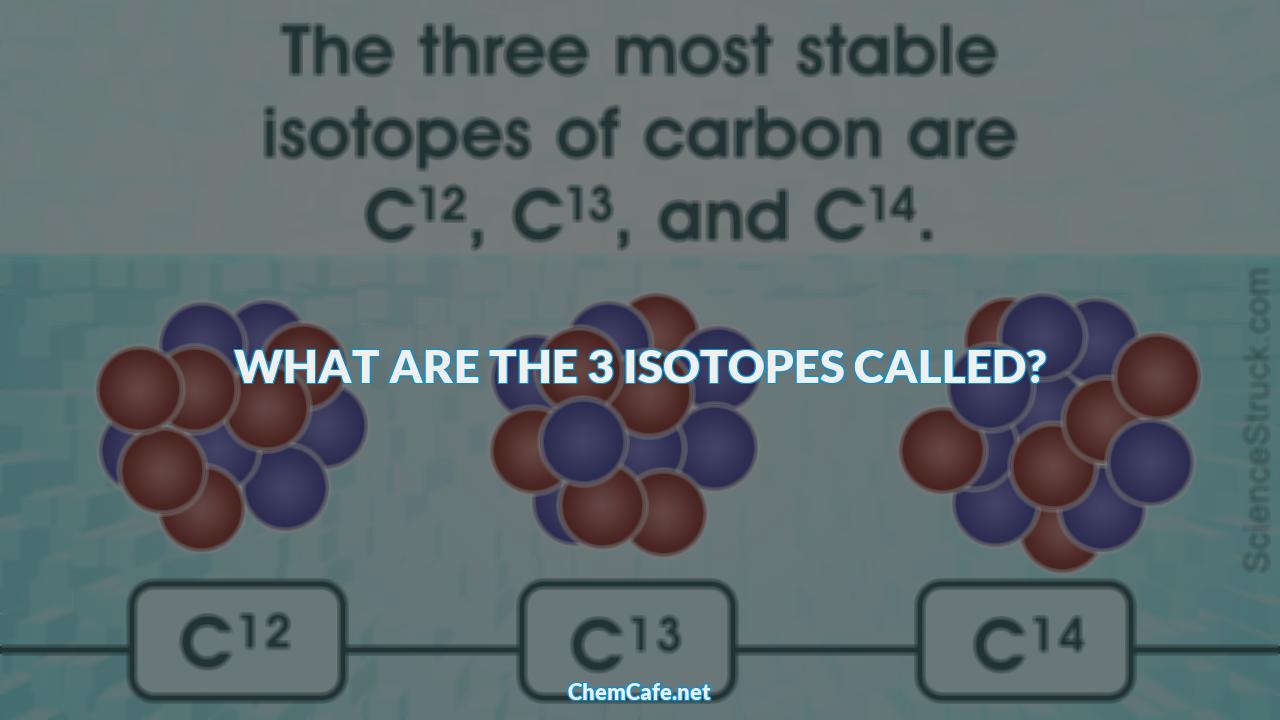
One of the most important elements that has multiple isotopes is carbon. Carbon has three naturally occurring isotopes, which are carbon-12, carbon-13, and carbon-14. All three of these isotopes have the same atomic number (Z=6), meaning that they all have 6 protons in their nucleus, but they have different atomic masses (carbon-14 being the heaviest). This means that, chemically, all three are indistinguishable.
However, some isotopes have the ability to transform into another element entirely. This is because of the differences in mass and nuclear structure that exist between the isotopes. As an example, hydrogen only has three isotopes, which are hydrogen-1, hydrogen-2, and hydrogen-3. Each of these isotopes has one proton in its nucleus, but hydrogen-2 and hydrogen-3 also have one and two neutrons, respectively. This results in hydrogen-2 having an atomic mass of two and hydrogen-3 having an atomic mass of three.
These isotopes all have different properties, which makes them useful for a variety of applications. For instance, hydrogen-2, or deuterium, is used as a fuel source in nuclear fusion, while hydrogen-3, or tritium, is used as an energy source in nuclear weapons.
Overall, isotopes are a unique form of atoms that can have different properties and uses. Despite their differences, all isotopes of the same element have the same atomic number, which makes them chemically indistinguishable. Carbon, hydrogen, and uranium are just a few of the elements that have multiple isotopes, each of which have their own unique properties and applications.
What are the 3 isotopes called?
Isotopes are atoms of the same element that have different numbers of neutrons. Three of the most common isotopes are carbon-12, carbon-13 and carbon-14, which are all naturally occurring.
What Is an Isotope?
An isotope is one of two or more species of atoms of a chemical element with the same atomic number and position in the periodic table and nearly identical chemical behavior but with different atomic masses and physical properties. Every chemical element has one or more isotopes.
What Causes the Differences Among Isotopes?
Differences in the properties of isotopes can be attributed to either of two causes: differences in mass or differences in nuclear structure. In the standard notation, 11H refers to the simplest isotope of hydrogen and 23592U to an isotope of uranium widely used for nuclear power generation and nuclear weapons fabrication. (Authors who do not wish to use symbols sometimes write out the element name and mass number—hydrogen-1 and uranium-235 in the examples above.)
The term nuclide is used to describe particular isotopes, notably in cases where the nuclear rather than the chemical properties of an atom are to be emphasized.
The Three Commonly Occurring Carbon Isotopes
The three commonly occurring carbon isotopes are carbon-12, carbon-13, and carbon-14. All three isotopes have the same atomic number (Z=6), which means that they all have six protons in their nuclei.
Carbon-12 is the most abundant of the three isotopes, making up 99.98% of the carbon on Earth. It has six protons and six neutrons, giving it an atomic mass of 12. Carbon-13 has seven neutrons, giving it an atomic mass of 13 and making it 0.01% of the total carbon on Earth. The third isotope, carbon-14, has eight neutrons and an atomic mass of 14, making it 0.0000000001% of the total carbon on Earth.
Are All Isotopes Identical Chemically?
No. All three isotopes of carbon have different atomic masses (carbon-14 being the heaviest), but share the same atomic number (Z=6). Chemically, all three are indistinguishable, because the number of electrons in each of these three isotopes is the same. So different isotopes of the same element are identical, chemically speaking. But some isotopes have the ability to circumvent this rule by transforming into another element entirely.
For example, carbon-14 is radioactive. It is unstable and decays into nitrogen-14 with a half-life of approximately 5700 years. This process is known as radioactive decay and is the basis of radiocarbon dating.
In conclusion, isotopes are atoms of the same element that have different numbers of neutrons. Three of the most common isotopes are carbon-12, carbon-13 and carbon-14, which are all naturally occurring. These three isotopes have different atomic masses, but share the same atomic number. All three isotopes are chemically indistinguishable from each other, but some isotopes have the ability to transform into another element entirely.
What are the 3 types of isotopes?
Isotopes are the variants of a particular element that have the same atomic number but a different atomic mass. This is due to the unequal number of neutrons in the atom. Isotopes are classified into two main types: stable and radioactive. Knowing the differences between these two helps us to distinguish one isotope from another.
Stable Isotopes
Stable isotopes are those which do not undergo any radioactive decay. They are also known as stable nuclides. The number of stable isotopes that are naturally occurring on the planet earth is around 339. These isotopes are chemically and physically similar to their parent element and can be found in abundance in nature.
The most abundant stable isotope is hydrogen-1 with a relative atomic mass of 1.008. It accounts for more than 99% of all hydrogen atoms on Earth. Other than hydrogen, the most abundant stable isotopes are carbon-12 and oxygen-16, with relative atomic masses of 12 and 16 respectively.
Radioactive Isotopes
Radioactive isotopes are those which decay over time and emit radiation. This is called radioactivity. These isotopes are also known as radioisotopes or radionuclides. All of them have unstable nuclei, which means that they are constantly undergoing nuclear decay. This decay results in the release of energy in the form of particles or radiation.
The most common radioactive isotopes are uranium-238 and uranium-235, which have relative atomic masses of 238 and 235 respectively. These two isotopes are the main source of nuclear energy. Other radioactive isotopes include carbon-14, iodine-131, and cobalt-60.
Artificial Isotopes
In addition to naturally occurring isotopes, scientists can also create isotopes in the lab. These are called artificial isotopes. This is usually done by bombarding a stable isotope with a beam of particles, such as protons or neutrons. This results in a change in the number of neutrons in the atom and, hence, the atomic mass.
The most commonly used artificial isotopes are carbon-11, fluorine-18, and phosphorus-32. These isotopes are used in medical imaging, as they emit radiation which can be detected by special cameras. They are also used in cancer treatment, as they can be used to target and destroy cancer cells.
In conclusion, there are three types of isotopes: stable, radioactive, and artificial. Stable isotopes are abundant in nature and do not undergo any decay. Radioactive isotopes decay over time and emit radiation. Artificial isotopes are created in the lab and are used in medical imaging and cancer treatment. Understanding the differences between these isotopes helps us to identify and distinguish them.
Does hydrogen have 3 isotopes?
Yes, hydrogen has three naturally occurring isotopes – 1H, 2H, and 3H. Although scientists have synthesized more highly unstable nuclei such as 4H to 7H in the laboratory, they are not found in nature.
The Most Common Hydrogen Isotope
1H is the most abundant hydrogen isotope, comprising more than 99.98% of hydrogen atoms found in nature. It is given the formal name of protium, due to its single proton nucleus.
What Are Isotopes?
Think of isotopes as different versions of an element. They are all the same element, meaning they all have the same atomic number, but differ in the number of neutrons. This difference in neutrons changes the atomic mass, which is the total number of protons and neutrons combined.
Hydrogen’s Three Isotopes
Hydrogen has three isotopes: protium or hydrogen-1, deuterium or hydrogen-2, and tritium or hydrogen-3.
Protium, also known as ordinary hydrogen, is the lightest isotope. It is followed by deuterium, also known as heavy hydrogen. Tritium, on the other hand, has trace amounts and is found in nature only in small amounts.
The Uses of Hydrogen Isotopes
Hydrogen isotopes are used in a variety of applications. For instance, protium is used in the production of ammonia and methanol, while deuterium is used in medical imaging and in the production of heavy water. Tritium is employed in nuclear reactors as a source of fuel.
Hydrogen has three naturally occurring isotopes: 1H, 2H, and 3H. Each isotope has a different number of neutrons, which results in a different atomic mass. Protium, deuterium, and tritium are all used for various purposes, from industrial production to nuclear energy.
What are the three isotopes of hydrogen and how do they differ quizlet?
Hydrogen is the lightest and most abundant element in the universe, and it is composed of three different isotopes—protium, deuterium, and tritium. Each of these isotopes has a different number of neutrons, resulting in different atomic masses and properties.
Protium (Hydrogen-1)
Protium, also known as ordinary hydrogen, is the most abundant isotope of hydrogen, making up 99.98% of natural hydrogen. It is the lightest isotope, having just one proton in its nucleus. This single-proton nucleus gives protium the formal name of “protium”.
Deuterium (Hydrogen-2)
Deuterium, also known as heavy hydrogen, is the second most abundant isotope of hydrogen, making up about 0.02% of natural hydrogen. It has a nucleus containing one proton and one neutron, and its atomic mass is twice that of protium.
Tritium (Hydrogen-3)
Tritium, also known as superheavy hydrogen, is the least abundant isotope of hydrogen, with a natural abundance of less than one-billionth of a percent. It has a nucleus containing one proton and two neutrons, and its atomic mass is three times that of protium. Tritium is unstable and radioactive, with a half-life of 12.3 years.
Differences between the Isotopes of Hydrogen
The different isotopes of hydrogen have different properties due to the difference in their atomic masses. The lightest isotope, protium, is the most abundant and the most stable. Deuterium is twice as heavy as protium, and is also stable. Tritium is three times as heavy as protium, and is radioactive, with a relatively short half-life.
Each isotope has a different range of applications. Protium is used in a wide variety of industrial and scientific applications, such as hydrogen fuel cells and hydrogen-oxygen rockets. Deuterium is used in the production of heavy water, which is used in nuclear reactors. Tritium is used in the production of tritium-labeled compounds, which are used in medical imaging and diagnostics.
The differences between the isotopes of hydrogen are important to understand when considering the properties and applications of hydrogen. Protium, deuterium, and tritium all have different atomic masses and properties, and each has a wide range of applications. By understanding the differences between the isotopes of hydrogen, we can better understand the properties and applications of hydrogen.
What differentiates the isotopes of hydrogen H 1 H 2 and H 3?
The three naturally-occurring isotopes of hydrogen are protium, deuterium, and tritium. Each isotope has its own unique properties, and all three are commonly used for dating. There are also four to seven other hydrogen isotopes that can be created in the laboratory. The least stable isotope is 7H and the most stable is 5H. The most stable radioactive isotope is tritium.
What are the Isotopes of Hydrogen?
Hydrogen is the simplest element and therefore has been studied extensively. The isotopes of hydrogen show many of the same effects found in more complex nuclei. An isotope is a nucleus with the same atomic number (Z) but a different mass number (A).
The three isotopes of hydrogen are protium, deuterium, and tritium. These three isotopes can be distinguished from one another by the number of neutrons they possess. Protium has no neutrons, deuterium has one, and tritium has two. Each of the three isotopes has a mass number of one, two, and three respectively, and the corresponding nuclear symbols are 1H, 2H, and 3H. All three isotopes have one electron to balance the charge of the single proton.
Protium
The most common isotope of hydrogen is protium, or 1H. Protium is the lightest of the three isotopes, and it is the only stable, non-radioactive isotope of hydrogen. In nature, 99.985% of all hydrogen atoms are protium. It is the most common form of hydrogen found in the universe.
Protium is used in a variety of applications, including the production of nuclear power and the manufacture of chemical products. It is also used in medical diagnostic tests and in the treatment of certain diseases.
Deuterium
Deuterium, or 2H, is the second most common isotope of hydrogen. It is also referred to as “heavy hydrogen” due to its larger mass compared to protium. Deuterium is a stable, non-radioactive isotope, and it is found in nature in a proportion of about 0.015%.
Deuterium has many uses, including the production of tritium, a radioactive isotope used in the making of nuclear weapons. It is also used in the manufacture of pharmaceuticals and in medical research.
Tritium
Tritium, or 3H, is the least common of the three isotopes of hydrogen. It is also the most unstable and radioactive isotope. Tritium is found in nature in very small quantities, and it has a half-life of 12.3 years.
Tritium is used in a variety of applications, including the production of nuclear weapons, the manufacture of luminous paints and inks, and medical research. It is also used in the production of tritium-enriched water, which is used in nuclear reactors.
FAQs
Q: What is the most stable isotope of hydrogen?
A: The most stable isotope of hydrogen is tritium.
Q: What is the least stable isotope of hydrogen?
A: The least stable isotope of hydrogen is 7H.
Q: What are the uses of the isotopes of hydrogen?
A: The isotopes of hydrogen have a variety of uses, including the production of nuclear power and weapons, the manufacture of chemical products, medical research, and the production of tritium-enriched water.
Why does hydrogen only have 3 isotopes?
Hydrogen is one of the most abundant elements in the universe, but did you know that it only has three naturally occurring isotopes? Isotopes are different versions of the same element, with the same atomic number but different number of neutrons. This slight difference in the number of neutrons changes the atomic mass, resulting in three different isotopes of hydrogen.
The Three Isotopes of Hydrogen
The three isotopes of hydrogen are protium (1H), deuterium (2H) and tritium (3H). Protium, also known as ordinary hydrogen, is the most common hydrogen isotope, making up more than 99.98% of hydrogen found in nature. It has a single proton in its nucleus, which gives it its formal name of protium. Deuterium, also known as heavy hydrogen, has a single neutron in its nucleus. It is much rarer than protium and only makes up 0.02% of naturally occurring hydrogen. The third isotope is tritium, which has two neutrons in its nucleus. Tritium is extremely rare and is only found in trace amounts in nature.
Synthesized Isotopes of Hydrogen
In addition to the three naturally occurring isotopes of hydrogen, other, highly unstable nuclei have been synthesized in the laboratory. These isotopes are numbered from 4H to 7H, but are not observed in nature. The synthesis of these isotopes is a complex process that involves accelerating a particle, such as a proton, to near the speed of light, and then colliding it with a target. This process is used to create new isotopes of elements, such as hydrogen, and study their properties.
Uses of Hydrogen Isotopes
Hydrogen isotopes are used in a variety of applications, from medical research to nuclear energy. Protium, the most common hydrogen isotope, is used as a fuel source in some types of nuclear reactors. Deuterium is used in medical research as a tracer to track the flow of molecules in the body. Tritium is used in self-luminous lighting and nuclear weapons.
Hydrogen is one of the most abundant elements in the universe, but it only has three naturally occurring isotopes: protium, deuterium and tritium. Protium is the most common isotope and is used as a fuel source in some types of nuclear reactors. Deuterium is used in medical research, while tritium is used in self-luminous lighting and nuclear weapons. Other, highly unstable nuclei have been synthesized in the laboratory, but are not found in nature. Understanding the properties of isotopes is essential for a wide range of applications.




Leave a Comment