Welcome to the world of elements! Have you ever wondered what element is 1s2 2s2 2p6 3s2 3p6 3d10 4s2 4p6? Or what element is 1s2 2s2 2p6 3s2 3p6 4s2 3d3? What about the elements of 1s 2s 2p 3s 3p?
In this blog post, we will explore the world of elements, starting with the element of 1s2 2s2 2p6 3s2 3p6 3d10 4s2 4p6. This element is called Iridium and has an atomic weight of 192.2 and atomic number of 77. It is a transition element, meaning that it can move between several energy levels in its outer orbit. Its electron configuration is 1s2 2s2 2p6 3s2 3p6 4s2 3d10 4p6 5s2 4d10 5p6 6s2 4f14 5d7.
The next element we will look at is 1s2 2s2 2p6 3s2 3p6 4s2 3d3. This element is called Tantalum and has an atomic weight of 180.9 and atomic number of 73. It is also a transition element, with an electron configuration of 1s2 2s2 2p6 3s2 3p6 4s2 3d10 4p6 5s2 4d10 5p6 6s2 4f14 5d3.
Now, let’s take a look at the element of 1s 2s 2p 3s 3p. This element is called Rhodium and has an atomic weight of 102.9 and atomic number of 46. It is also a transition element, with an electron configuration of 1s2 2s2 2p6 3s2 3p6 4s2 3d7.
The next element we will look at is 1s2 2s2 2p6 3s2 3p6 4s2 3d10. This element is called Palladium and has an atomic weight of 106.4 and atomic number of 47. It is also a transition element, with an electron configuration of 1s2 2s2 2p6 3s2 3p6 4s2 3d10 4p6 5s2 4d8.
Finally, let’s take a look at the element of 1s2 2s2 2p6 3s2 3p6 4s2 3d1 3d1 3d1. This element is called Uranium and has an atomic weight of 238.0 and atomic number of 92. It is a transition element, with an electron configuration of 1s2 2s2 2p6 3s2 3p6 4s2 3d1 3d1 3d1.
Now that you have a better understanding of the elements and their electron configurations, you can more confidently answer the questions, “What element is 1s2 2s2 2p6 3s2 3p6 3d10 4s2 4p6?” and “What element is 1s2 2s2 2p6 3s2 3p6 4s2 3d3?”
Thanks for joining us on this exploration of the world of elements, and we hope that you have a better understanding of the element of 1s2 2s2 2p6 3s2 3p6 3d10 4s2 4p6 and its electron configuration!
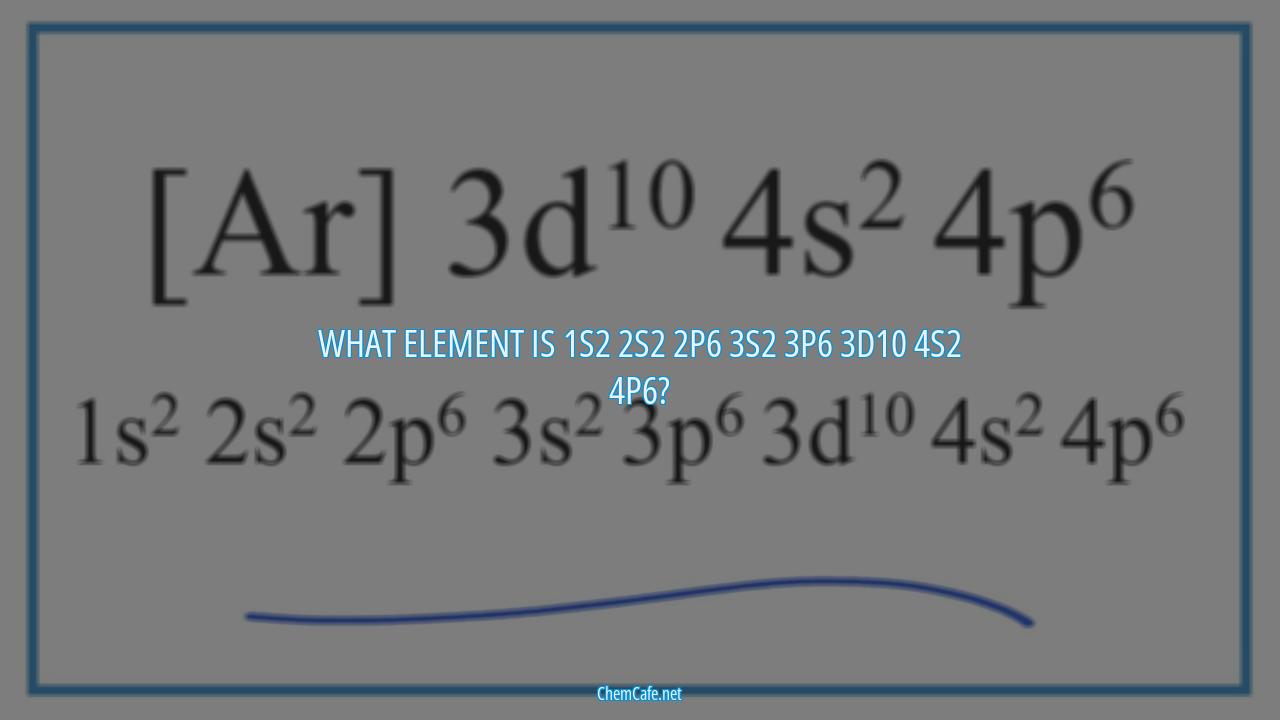
What element is 1s2 2s2 2p6 3s2 3p6 3d10 4s2 4p6?
The electron configuration of an element determines its chemical properties and is an important factor in determining its identity. It is a representation of the arrangement of electrons around the nucleus of an atom. In this article, we will discuss the element with the electron configuration 1s2 2s2 2p6 3s2 3p6 3d10 4s2 4p6.
The electron configuration 1s2 2s2 2p6 3s2 3p6 3d10 4s2 4p6 is the electron configuration of the element iridium, which has an atomic number of 77. Iridium is a transition metal and is one of the most corrosion-resistant elements on the planet. It is a hard, brittle, silvery-white metal that is found in many alloys and is used in a variety of industries, including electronics, automotive, and medical.
Atomic Properties of Iridium
Iridium has an atomic weight of 192.2 and a melting point of 2466°C. It is a relatively rare element, occurring at a concentration of 0.001 parts per million in the Earth’s crust. It is also the most dense of all elements, with a density of 22.56 g/cm3. Iridium is generally considered to be a noble metal, meaning that it is not easily oxidized in air and is not affected by most acids.
Uses of Iridium
Iridium is a valuable metal for a variety of applications. It is used in the manufacture of electrical contacts, jewelry, and medical instruments. It is also used as a catalyst in the manufacture of pharmaceuticals, as a coating for laboratory glassware, and as an alloying element in the production of alloys.
Reactivity of Iridium
Iridium is a relatively unreactive element. It is not affected by air or water, although it can be oxidized by hot concentrated acids. It is also unaffected by most other chemicals, making it an excellent choice for applications where corrosion resistance is required.
Safety of Iridium
Iridium is considered to be a safe element to work with. It is non-toxic and has no known adverse effects on human health. It is also not flammable, making it a safe choice for many industrial applications.
In conclusion, the element with the electron configuration 1s2 2s2 2p6 3s2 3p6 3d10 4s2 4p6 is iridium. Iridium is a transition metal that is relatively unreactive and is used in a variety of industries. It is also a safe element to work with and has no known adverse effects on human health.
What element is 1s2 2s2 2p6 3s2 3p6 4s2 3d3?
When looking at the electronic configuration of an element, it can be quite confusing. This is because it is written in a specific format that uses symbols to represent the electrons in the element’s shells. The electronic configuration of an element is an important tool for chemists, as it can be used to identify the element and determine its properties.
In this article, we will discuss what element is 1s2 2s2 2p6 3s2 3p6 4s2 3d3. We will also explore the periodic table and the role of electronic configurations in chemistry.
What Does 1s2 2s2 2p6 3s2 3p6 4s2 3d3 Represent?
The 1s2 2s2 2p6 3s2 3p6 4s2 3d3 electronic configuration belongs to the element tantalum. Tantalum is a transition metal located in group 5 of the periodic table. Its atomic number is 73 and its atomic weight is 180.9.
The electronic configuration of an element is written in a specific format. The 1s2 2s2 2p6 3s2 3p6 4s2 3d3 notation indicates that the element has two electrons in its first shell, two electrons in its second shell, six electrons in its third shell, two electrons in its fourth shell, six electrons in its fifth shell, two electrons in its sixth shell, and three electrons in its seventh shell.
What is the Role of Electronic Configurations in Chemistry?
The electronic configuration of an element is important in chemistry as it can be used to identify the element and determine its properties. The electronic configuration of an element can be used to determine the element’s reactivity, its stability, and its position in the periodic table. It can also be used to predict the element’s bonding behavior and its reactivity with other elements.
For example, the electronic configuration of sodium is 1s2 2s2 2p6 3s1. This indicates that sodium has one electron in its outermost shell, making it highly reactive. It also has a single electron in its outermost shell, which makes it very reactive with other elements and allows it to form strong bonds.
Other Elements with a Similar Electronic Configuration
The 1s2 2s2 2p6 3s2 3p6 4s2 3d3 electronic configuration belongs to the element tantalum. However, there are other elements with a similar electronic configuration. These elements are iridium, rhodium, and palladium.
Iridium has an atomic number of 77 and an atomic weight of 192.2. Its electronic configuration is 1s2 2s2 2p6 3s2 3p6 4s2 3d10 4p6 5s2 4d10 5p6 6s2 4f14 5d7.
Rhodium has an atomic number of 46 and an atomic weight of 102.9. Its electronic configuration is 1s2 2s2 2p6 3s2 3p6 4s2 3d10 4p6 5s2 4d7.
Palladium has an atomic number of 47 and an atomic weight of 106.4. Its electronic configuration is 1s2 2s2 2p6 3s2 3p6 4s2 3d10 4p6 5s2 4d8.
The 1s2 2s2 2p6 3s2 3p6 4s2 3d3 electronic configuration belongs to the element tantalum. It is a transition metal located in group 5 of the periodic table and has an atomic number of 73 and an atomic weight of 180.9. The electronic configuration of an element is important in chemistry as it can be used to identify the element and determine its properties. Other elements with a similar electronic configuration include iridium, rhodium, and palladium.
What is the element of 1s 2s 2p 3s 3p?
The element of 1s 2s 2p 3s 3p is a configuration used to describe the electron arrangement of atoms in the periodic table. It is the sum of the electrons in each of the s and p orbitals, and it indicates how many electrons an atom has in its outermost shell.
The 1s orbital is the first orbital and has one electron, while the 2s and 2p orbitals each have two electrons. The 3s and 3p orbitals have three electrons each. This configuration is often written as 1s2 2s2 2p3 3s3 3p3.
When looking at the periodic table, you can find the element of 1s 2s 2p 3s 3p by looking at the element’s position in the table. Elements in the first row, for example, have one electron in the 1s orbital and two electrons in the 2s orbital. Elements in the second row have two electrons in the 2s orbital, and five electrons in the 2p orbital.
For example, element A has two valence electrons in 2s (2s2) and five valence electrons in 2p (2p5). This means the element of 1s 2s 2p 3s 3p for element A is 2s22p5. It has 2 + 5 = 7 valence electrons.
Element B is located in Period 3, the 2nd position in 3s-block. This means that B has two valence electrons in 3s (3s2). Therefore, the element of 1s 2s 2p 3s 3p for element B is 3s2.
Element C is located in Period 5, the 1st position in 5s-block). This means that there is only one valence electron in 5s (5s1). Thus, the element of 1s 2s 2p 3s 3p for element C is 5s1.
This is followed by the second row p-block, containing 6 elements (B through Ne) since six electrons are required to fill the 2p subshell. The third row is similar to the second row elements. Two electrons are needed (Na and Mg) to fill the 3s subshell and six electrons are required (Al through Ar) to complete the 3p subshell.
Therefore, the electron configuration of neutral chlorine atoms is 1s22s22p63s23p5. This means that there are two electrons in the 1s subshell, two electrons in the 2s subshell, and six electrons in the 2p subshell. This is followed by two electrons in the 3s subshell, and five electrons in the 3p subshell.
In conclusion, the element of 1s 2s 2p 3s 3p describes the electron arrangement of atoms in the periodic table. This configuration is used to determine the number of electrons each atom has in its outermost shell. The element of 1s 2s 2p 3s 3p for a particular element can be determined by looking at its position in the periodic table.
What element is 1s2 2s2 2p6 3s2 3p6 4s2 3d10?
Atoms are made up of various elements, each with their own unique properties. Each element has an atomic number and electron configuration which can help to distinguish one from the other.
When looking at the electron configuration of an element, it is important to understand what each part of the configuration means. For example, the configuration 1s2 2s2 2p6 3s2 3p6 4s2 3d10 indicates that the element has seven valence electrons. Valence electrons are electrons located in the outermost shell of an atom and are responsible for its chemical properties.
What is 1s2 2s2 2p6?
The first part of the electron configuration, 1s2 2s2 2p6, indicates that the element has two valence electrons in 2s (2s2) and five valence electrons in 2p (2p5). This means that the element A has seven valence electrons in total (2 + 5 = 7).
What is 3s2 3p6 4s2 3d10?
The second part of the electron configuration, 3s2 3p6 4s2 3d10, indicates that the element B is located in Period 3, the 2nd position in 3s-block. This means that B has two valence electrons in 3s (3s2).
The third part of the electron configuration, 4s2 3d10, indicates that the element C is located in Period 5, the 1st position in 5s-block. This means that there is only one valence electron in 5s (5s1).
What element is 1s2 2s2 2p6 3s2 3p6 4s2 3d10?
The electron configuration 1s2 2s2 2p6 3s2 3p6 4s2 3d10 indicates that the element is Tantalum (Ta) with an atomic weight of 180.9 and an atomic number of 73. This element is classified as a transition element, meaning it can easily form bonds and transfer electrons.
Another element with a similar electron configuration is Iridium (Ir) with an atomic weight of 192.2 and an atomic number of 77. This element also belongs to the transition elements group. Its electron configuration is 1s2 2s2 2p6 3s2 3p6 4s2 3d10 4p6 5s2 4d10 5p6 6s2 4f14 5d7.
In conclusion, the element with the electron configuration 1s2 2s2 2p6 3s2 3p6 4s2 3d10 is Tantalum (Ta). This element has seven valence electrons and is classified as a transition element. Iridium (Ir) is another element with a similar electron configuration. Understanding the electron configuration of an element can help to identify its properties and distinguish it from other elements.
What element is 1s2 2s2 2p6 3s2 3p6?
Have you ever been confused by electronic configuration notation? You’re not alone – it’s a confusing topic, and a lot of people struggle to understand it. To help you out, we’ll be delving into what element is 1s2 2s2 2p6 3s2 3p6.
The Basics of Electronic Configuration
Electronic configuration notation is a way of representing the arrangement of electrons in an atom. It’s usually written using the symbol for the element followed by a series of numbers and letters. For example, the electronic configuration for nitrogen is 1s2 2s2 2p3.
The first number (1 in this case) represents the energy level of the electrons. The letter (s in this case) represents the sublevel of the electrons. The second number (2 in this case) represents the number of electrons in the sublevel.
What About 1s2 2s2 2p6 3s2 3p6?
So, what element is 1s2 2s2 2p6 3s2 3p6? This electronic configuration belongs to chlorine, a member of Group 17 (or the halogen group) on the periodic table. Chlorine has two electrons in the 1s sublevel (1s2), two electrons in the 2s sublevel (2s2), six electrons in the 2p sublevel (2p6), two electrons in the 3s sublevel (3s2), and six electrons in the 3p sublevel (3p6).
Other Elements of Interest
If you’re still learning about electronic configuration notation, you might be interested in some other elements as well.
For example, the electronic configuration of titanium (Ti) is 1s2 2s2 2p6 3s2 3p6 3d2 4s2. This means that titanium has two electrons in the 1s sublevel, two electrons in the 2s sublevel, six electrons in the 2p sublevel, two electrons in the 3s sublevel, six electrons in the 3p sublevel, two electrons in the 3d sublevel, and two electrons in the 4s sublevel.
Neon (Ne) has an electronic configuration of 1s2 2s2 2p6. This means that neon has two electrons in the 1s sublevel, two electrons in the 2s sublevel, and six electrons in the 2p sublevel.
Finally, Group 4A of the periodic table includes the nonmetal carbon (C), the metalloids silicon (Si) and germanium (Ge), the metals tin (Sn) and lead (Pb), and the yet-unnamed artificially-produced element ununquadium (Uuq).
We hope this article has been helpful in understanding the electronic configuration notation of elements. The element with an electronic configuration of 1s2 2s2 2p6 3s2 3p6 is chlorine, which belongs to Group 17 (or the halogen group) on the periodic table. Other elements of interest include titanium (Ti), neon (Ne), and Group 4A of the periodic table.
Understanding electronic configuration notation is an important part of understanding chemistry and the periodic table, so we hope this article has been useful in helping you learn more about the topic. Good luck!
What element is 1s2 2s2 2p6 3s2 3p6 4s2 3d1 3d1 3d1?
The element in question is tantalum (Ta). It is a transition metal located in the 5th period of the periodic table. It has an atomic number of 73 and an atomic weight of 180.9. Its electron configuration is 1s2 2s2 2p6 3s2 3p6 4s2 3d10 4p6 5s2 4d10 5p6 6s2 4f14 5d3.
Tantalum is a silvery-gray metal that is highly resistant to corrosion. It is also an excellent conductor of heat and electricity. It has a melting point of 3017°C (5465°F) and a boiling point of 5458°C (9854°F). It is used in a wide range of industrial applications including electronics, aerospace, and medical equipment.
Tantalum is a relatively rare element. It is found in the earth’s crust in small amounts, usually in combination with other metals. It is produced commercially by the electrolysis of molten alkali metal chlorides. It is also found in minerals such as tantalite, columbite, and microlite.
The electron configuration of 1s2 2s2 2p6 3s2 3p6 4s2 3d1 3d1 3d1 is the same as the electron configuration of iridium (Ir), another transition metal. Iridium has an atomic number of 77 and an atomic weight of 192.2. Its electron configuration is 1s2 2s2 2p6 3s2 3p6 4s2 3d10 4p6 5s2 4d10 5p6 6s2 4f14 5d7.
Iridium is a very hard, brittle metal that is highly resistant to corrosion. It has a melting point of 2410°C (4390°F) and a boiling point of 4428°C (7992°F). It is used in a variety of industrial and medical applications, such as in spark plugs, surgical instruments, and X-ray tubes.
Iridium is also a rare element. It is found in the earth’s crust in very small amounts, usually in combination with other metals. It is produced commercially by the electrolysis of molten alkali metal chlorides. It is also found in minerals such as iridosmine, osmiridium, and rutheniridosmine.
In conclusion, 1s2 2s2 2p6 3s2 3p6 4s2 3d1 3d1 3d1 is the electron configuration of two transition metals: tantalum (Ta) and iridium (Ir). Both of these elements are relatively rare and have a wide range of industrial and medical applications.



Leave a Comment