Have you ever wondered what an atom looks like? Or how electrons, protons, and neutrons interact within an atom? All of these fascinating questions can be answered by understanding the electron configuration of atoms. The electron configuration of an atom refers to the arrangement of electrons in the different energy levels and orbitals of the atom. It is this arrangement that determines the atom’s chemical and physical properties.
In this article, we will discuss the electron configuration of atoms, the rarest chemical on earth, elements 0 and beyond, the newest elements, if unknown elements exist, and the super rarest elements. We will also provide a table summarizing the electron configurations of the first 18 elements.
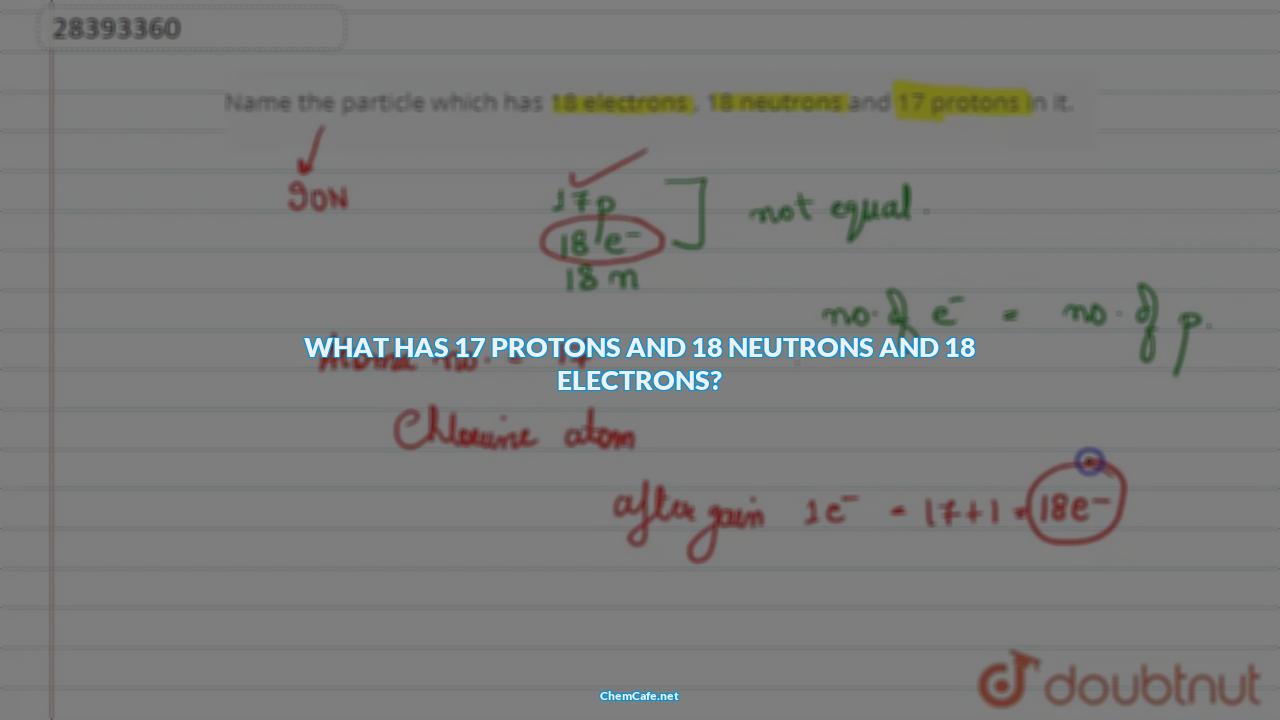
Let’s start by looking at what has 17 protons and 18 neutrons and 18 electrons. This atomic configuration is found in the element helium, which has two electrons. The electrons are assigned to the 1s sublevel, the lowest-energy sublevel in the lowest-energy level. Therefore, the electron configuration of helium is: He: 1s2. This means that the two electrons completely fill the first energy level.
In addition to helium, elements with higher atomic numbers such as lithium, boron, carbon, nitrogen, oxygen, fluorine, neon, sodium, magnesium, aluminum, silicon, phosphorus, sulfur, chlorine, and argon also have unique electron configurations that can be determined using the orbital-filling chart in Figure 5.9.
Furthermore, box diagrams can also be used to show the distribution of electrons among the orbitals. Unpaired electrons (a single electron in an orbital) are in a lower energy configuration than are paired electrons (two electrons in an orbital).
Finally, let’s take a look at the rarest chemical on earth, element 0, the newest elements, and the super rarest elements. Astatine (At) may be the rarest naturally occurring element in the Earth’s crust, but it is a member of the halogen family. Neutronium is the hypothetical element zero, with no protons in its atomic nucleus. The fourth element is named Oganesson, after a Russian nuclear physicist named Yuri Oganessian.
As you can see, the electron configuration of atoms is an incredibly important concept in chemistry. Understanding the electron configuration of atoms allows us to understand their chemical and physical properties.
What has 17 protons and 18 neutrons and 18 electrons?
Understanding how electrons move around atoms is essential to understanding chemistry. Electrons can be found in different energy levels, or shells, around the nucleus of an atom. The number of protons and neutrons in an atom determine the element, while the arrangement of electrons determines the chemical properties of the atom. In this article, we’ll take a look at what has 17 protons, 18 neutrons, and 18 electrons.
The Electron Configurations of Atoms
The electron configuration of an atom shows the number of electrons in each sublevel in each energy level of the ground-state atom. To determine the electron configuration of a particular atom, start at the nucleus and add electrons one by one until the number of electrons equals the number of protons in the nucleus. Each added electron is assigned to the lowest-energy sublevel available. The first sublevel filled will be the 1s sublevel, then the 2s sublevel, the 2p sublevel, the 3s, 3p, 4s, 3d, and so on. This order is difficult to remember and often hard to determine from energy-level diagrams such as Figure 5.8
A more convenient way to remember the order is to use Figure 5.9. The principal energy levels are listed in columns, starting at the left with the 1s level. To use this figure, read along the diagonal lines in the direction of the arrow. The order is summarized under the diagram.
What has 17 Protons and 18 Neutrons and 18 Electrons?
An atom of chlorine (Cl) has 17 protons, 18 neutrons and 18 electrons. Its electron configuration is 1s22s22p63s23p5. This means that the first shell has two electrons, the second shell has eight electrons, and the third shell has seven electrons.
The single electron is assigned to the 1s sublevel, the lowest-energy sublevel in the lowest-energy level. Therefore, the electron configuration of chlorine is written: Cl: 1s22s22p63s23p5. This means that the first shell has two electrons, the second shell has eight electrons, and the third shell has seven electrons.
What is the Rarest Chemical on Earth?
Astatine (At) may be the rarest naturally occurring element in the Earth’s crust, but it is a member of the halogen family [fluorine (F), chlorine (Cl), bromine (Br), iodine (I), and astatine (At)] and is presumed to have characteristics similar to other Group 17 elements. Astatine has 85 protons and 125 neutrons, and its electron configuration is 1s22s22p63s23p64s23d104p65s24d105p4.
Is Element 0 Possible?
Neutronium is the hypothetical element zero, with no protons in its atomic nucleus. Neutronium is the name of a theoretical element with atomic number 0 and symbol Nu that consists entirely of neutrons. Other names for neutronium are neutrium and neutrite. Although neutronium has never been observed, scientists believe that it could exist in the core of neutron stars.
What is the Newest Element?
The newest element on the periodic table is Oganesson (Og). It was discovered in 2002 and officially named in 2016. Its atomic number is 118, and it has 118 protons, 175 neutrons, and 118 electrons. Its electron configuration is 1s22s22p63s23p64s23d104p65s24d105p66s24f145d106p67s24f145d106p5.
Do Unknown Elements Exist?
Although there are elements we have not yet created or found in nature, scientists already know what they will be and can predict their properties. For example, element 125 has not been observed, but when it is, it will appear in a new row of the periodic table as a transition metal. It will have 125 protons, 181 neutrons, and 125 electrons, and its electron configuration will be 1s22s22p63s23p64s23d104p65s24d105p66s24f145d106p67s24f145d106p68s24f145d106p6.
What are the Super Rarest Elements?
The rarest elements on Earth are all radioactive, meaning they are unstable and break down over time. These include technetium (Tc), promethium (Pm), astatine (At), and francium (Fr). Technetium has 43 protons, 61 neutrons, and 43 electrons, and its electron configuration is 1s22s22p63s23p64s23d104p65s2. Promethium has 61 protons, 91 neutrons, and 61 electrons, and its electron configuration is 1s22s22p63s23p64s23d104p65s24d105p6. Astatine has 85 protons, 125 neutrons, and 85 electrons, and its electron configuration is 1s22s22p63s23p64s23d104p65s24d105p4. Francium has 87 protons, 133 neutrons, and 87 electrons, and its electron configuration is 1s22s22p63s23p64s23d104p65s24d105p66s1.
In conclusion, an atom with 17 protons, 18 neutrons, and 18 electrons is chlorine (Cl). Its electron configuration is 1s22s22p63s23p5. Astatine, promethium, technetium, and francium are the super rarest elements on Earth. They are all radioactive, meaning they are unstable and break down over time. Although there are elements we have not yet created or found in nature, scientists already know what they will be and can predict their properties.
What atom has 17 protons and 18 neutrons and 17 electrons?
Atoms are made up of three subatomic particles: protons, neutrons, and electrons. The number of protons and electrons in an atom determine its atomic number and the number of neutrons determine its mass number. An atom with 17 protons and 17 electrons is chlorine, an element found on the periodic table. Chlorine has three isotopes, which are atoms of the same element but with different numbers of neutrons. The most common isotope of chlorine has 17 protons, 18 neutrons, and 17 electrons.
Understanding Isotopes
Isotopes are atoms with the same number of protons but different numbers of neutrons. This means that atoms of the same element can have different atomic weights, or the sum of the number of protons and neutrons in the atom. For example, every chlorine atom has 17 protons and 17 electrons, but three out of four chlorine atoms have 18 neutrons and the fourth has 20 neutrons. These atoms have different atomic weights, 35 amu and 37 amu, respectively. The atomic weight of chlorine reported in the periodic table is an average of the two, at 35.5 amu.
Atomic Number and Ions
The atomic number of an element is the number of protons in the nucleus of the atom. It is one of the most important properties of an element and helps determine the mass number. The atomic number of chlorine is 17, since it has 17 protons in its nucleus. This also means that a chlorine atom has 17 electrons.
Ions are atoms with a charge, either positive or negative. The charge is determined by the number of protons and electrons in the atom. If an atom has an equal number of protons and electrons, it has a neutral charge. However, if an atom has more or fewer electrons than protons, it has a net charge.
Electron Configuration
The electron configuration of an element tells us the arrangement of electrons in the atom’s orbitals. The orbital-filling chart in Figure 5.9 can be used to determine the electron configuration of elements with higher atomic numbers. For example, the electron configuration of chlorine is 1s22s22p63s23p5, since it has 17 protons and 17 electrons.
Summary
Atoms are made up of three subatomic particles: protons, neutrons, and electrons. The atomic number of an element is the number of protons in the nucleus of the atom, and the atomic weight is the sum of the number of protons and neutrons. Isotopes are atoms with the same number of protons but different numbers of neutrons. A chlorine atom has 17 protons, 18 neutrons, and 17 electrons, and its atomic number is 17. The electron configuration of chlorine is 1s22s22p63s23p5.
What element has 17 protons 20 neutrons and 18 electrons?
Atoms are made up of protons, neutrons, and electrons and the number of these particles determines the properties of the element. The element with 17 protons, 20 neutrons, and 18 electrons is Chlorine (Cl). Chlorine is a Halogen element in Group 17, period 4 of the periodic table and is one of the most abundant elements on Earth.
Isotopes
Atoms of the same element can have different numbers of neutrons, forming isotopes. The atomic number of chlorine is 17, which means that all chlorine atoms have 17 protons. However, the number of neutrons can vary. For example, three out of four chlorine atoms weigh 35 amu (17 protons and 18 neutrons) and the fourth weighs 37 amu (17 protons and 20 neutrons). These are the isotopes of chlorine. The atomic weight reported in the periodic table is the average weight of 35.5 amu.
Ions
Atoms of the same element can also have a different number of electrons, forming ions. All atoms of chlorine (Cl) have 17 protons, but there are chlorine isotopes having 15 to 23 neutrons. Only two chlorine isotopes exist in significant amounts in nature, those with 18 neutrons (75.53% of all chlorine atoms found in nature), and those with 20 neutrons (24.47%). To write the symbol for an isotope, place the atomic number as a subscript and the mass number (protons plus neutrons) as a superscript to the left of the atomic symbol. The symbols for the two naturally occurring isotopes of chlorine then would be Cl17 and Cl20. In discussing these isotopes, we use the terms chlorine-35 and chlorine-37.
Stability
For a nucleus to be stable, the number of neutrons should (for the first few elements) equal or slightly exceed the number of protons. The table below shows the composition of typical atoms and ions.
Composition of Typical Atoms and Ions
| Electrons | Protons | Neutrons | Atomic Number | Atomic Weight (amu) | Total Charge (electron units) |
|---|---|---|---|---|---|
| 1 | 1 | 0 | 1 | 1.008 | 0 |
| 1 | 1 | 1 | 1 | 2.014 | 0 |
| 1 | 1 | 2 | 1 | 3.016 | 0 |
| 0 | 1 | 0 | 1 | 1.007 | +1 |
| 2 | 2 | 2 | 2 | 4.003 | 0 |
| 0 | 2 | 2 | 2 | 4.002 | +2 |
| 3 | 3 | 4 | 3 | 7.016 | 0 |
| 6 | 6 | 6 | 6 | 12.000 | 0 |
| 8 | 8 | 8 | 8 | 15.995 | 0 |
| 17 | 17 | 18 | 17 | 34.969 | 0 |
| 17 | 17 | 20 | 17 | 36.966 | 0 |
| 17 | 17 | 18 or 20 | 17 | 35.453 | 0 |
| 92 | 92 | 142 | 92 | 234.04 | 0 |
| 92 | 92 | 143 | 92 | 235.04 | 0 |
| 92 | 92 | 146 | 92 | 238.05 | 0 |
| 92 | 92 | varied | 92 | 238.03 | 0 |
Mass Loss
When an atom of an element is formed from protons, electrons, and neutrons, a small amount of mass is lost. This mass loss is due to the binding energy that is released when the particles come together to form the atom. The amount of mass loss depends on the number of protons and neutrons in the nucleus. For example, when an atom of carbon-12 is formed from protons, electrons, and neutrons, the mass that is lost is 0.341 amu.
Chlorine is an element with 17 protons, 20 neutrons, and 18 electrons. It is a Halogen element in Group 17, period 4 of the periodic table and is one of the most abundant elements on Earth. Chlorine has two naturally occurring isotopes, those with 18 neutrons (75.53% of all chlorine atoms found in nature), and those with 20 neutrons (24.47%). When an atom of an element is formed from protons, electrons, and neutrons, a small amount of mass is lost due to the binding energy that is released when the particles come together to form the atom.
What has 17 protons and 18 electrons and 18 neutrons?
Isotopes of Chlorine
Atoms of each element consist of protons, electrons, and neutrons. The number of protons is the same for all atoms of a particular element, while the number of neutrons and electrons can vary. This variation gives rise to the formation of isotopes. Isotopes are atoms of the same element with different numbers of neutrons, and their various combinations of protons and neutrons determine their atomic weights.
Chlorine is one such element with isotopes. All chlorine atoms have 17 protons and 17 electrons, but the number of neutrons can vary. Out of four chlorine atoms, three weigh 35 amu (17 protons and 18 neutrons) and the fourth weighs 37 amu (17 protons and 20 neutrons). These are the isotopes of chlorine, and their average weight is reported in the periodic table as 35.5 amu.
Ions
Atoms of a particular element may also differ in their number of neutrons, forming isotopes. For instance, there are four isotopes of helium, which all have two protons but different numbers of neutrons. Similarly, chlorine atoms have 17 protons, but there are chlorine isotopes with 15 to 23 neutrons.
In nature, there are two chlorine isotopes that exist in significant amounts: those with 18 neutrons (75.53% of all chlorine atoms found in nature) and those with 20 neutrons (24.47%). To write the symbol for an isotope, the atomic number is placed as a subscript and the mass number (protons plus neutrons) is placed as a superscript to the left of the atomic symbol. The symbols for the two naturally occurring isotopes of chlorine are Cl and Cl.
Stability of Nuclei
For a nucleus to be stable, the number of neutrons should equal or slightly exceed the number of protons. This is the case for the first few elements. For example, in the case of hydrogen, the number of protons is 1, and the number of neutrons is 0. In the case of helium, there are two protons and two neutrons.
Composition of Typical Atoms and Ions
Table 1-2 gives the composition of typical atoms and ions. As one can see, the number of protons, electrons, and neutrons, as well as the atomic number, atomic weight, and total charge, are provided.
Example
Calculating the mass that is lost when an atom of carbon-12 is formed from protons, electrons, and neutrons is an example of how isotopes are used. The chlorine isotope has 17 protons and 20 neutrons. When the mass of the protons, electrons, and neutrons are added up, it is 37.3066 amu. The actual observed atomic weight of chlorine-37 is 36.966 amu, which means that 0.341 amu is lost in its formation.
Isotopes are atoms of the same element with different numbers of neutrons. Chlorine is an example of an element with isotopes, and there are two naturally occurring chlorine isotopes with 18 and 20 neutrons, respectively. The symbols for these isotopes are Cl and Cl. For a nucleus to be stable, the number of neutrons should equal or slightly exceed the number of protons. Table 1-2 gives the composition of typical atoms and ions, and an example of how isotopes are used to calculate mass losses upon atom formation is also given.
What element has 17 electrons?
A Closer Look at Electron Configuration
Atoms are the basic building blocks of matter. Atoms have a nucleus, which contains protons and neutrons, and a cloud of electrons that surround this nucleus. The number of protons and electrons in an atom determines its atomic number, and thus its place on the periodic table. Every atom of a given element has the same number of protons and electrons, which is determined by its atomic number. The element with 17 electrons, for example, is chlorine.
Understanding Electron Configuration
In order to understand why chlorine has 17 electrons, it’s important to first understand how electron configuration works. Electron configuration is the arrangement of electrons in an atom, which can be determined by looking at the orbitals of an atom.
The electrons in an atom occupy specific energy levels or orbitals. The orbitals are designated by the letters s, p, d, and f. The s orbital is the lowest energy level, followed by the p, d, and f orbitals. The number of electrons that can occupy a particular orbital is determined by the orbital’s energy level.
For example, the s orbital can hold up to two electrons, the p orbital can hold up to six electrons, the d orbital can hold up to 10 electrons, and the f orbital can hold up to 14 electrons.
Chlorine’s Electron Configuration
Chlorine is a chemical element with the atomic number of 17. This means that it has 17 protons and 17 electrons. Its electron configuration is written as 1s22s22p63s23p5. This means that the first two electrons occupy the 1s orbital, the next two occupy the 2s orbital, and the next six occupy the 2p orbital. The next three electrons occupy the 3s orbital, and the last five electrons occupy the 3p orbital.
The Importance of Electron Configuration
The electron configuration of an atom is important because it determines the chemical and physical properties of an element. For example, if two elements have the same number of electrons, but their electron configurations are different, they may have different chemical properties.
Understanding electron configuration is also important for understanding how atoms interact with one another. For example, when two atoms come into contact, they can form a bond by sharing electrons. The type of bond formed is determined by the electron configuration of the atoms involved.
Atoms are made up of protons, neutrons, and electrons. The number of electrons in an atom is determined by its atomic number, which is the same as the number of protons. The element with 17 electrons is chlorine, and its electron configuration is 1s22s22p63s23p5. Understanding electron configuration is important for understanding the chemical and physical properties of an element, as well as how atoms interact with one another.
What element has 17 protons 18 neutrons and 18 electrons?
Atoms are composed of protons, neutrons and electrons, and each element has a specific number of these particles. The element with 17 protons, 18 neutrons, and 18 electrons is chlorine. Chlorine is a non-metal element with an atomic number of 17 and an atomic weight of 35.453 g/mol.
Isotopes
Isotopes are atoms of the same element that have a different number of neutrons. Atoms of chlorine, for example, have 17 protons and 17 electrons, but can have either 18 or 20 neutrons. These different isotopes of chlorine have different atomic weights, with the common isotope having an atomic weight of 35 amu (atomic mass units) and the rare isotope having an atomic weight of 37 amu. The atomic weight reported for chlorine in the periodic table is the average atomic weight of both isotopes – 35.5 amu.
Ions
Ions are atoms with a net electrical charge due to the imbalance of protons and electrons. An atom of chlorine with 17 protons, 18 neutrons and 17 electrons has a neutral charge and is not an ion. The atomic number of chlorine is 17, which is the same as the number of protons in the nucleus of the atom.
Electron Configuration
The electron configuration of an element is the arrangement of electrons in the orbitals of the atom. The electron configuration of chlorine can be written using the orbital-filling chart in Figure 5.9. The electron configuration of chlorine is 1s22s22p63s23p5, which means that the chlorine atom has two electrons in the 1s orbital, two electrons in the 2s orbital, five electrons in the 2p orbital, and one electron in the 3s orbital.
Box Diagrams of Electron Configuration
Box diagrams can be used to show the distribution of electrons in an atom. Each box represents an orbital and the arrows within the boxes represent the electrons in that orbital. For example, the electron configuration of hydrogen can be represented by the following box diagram:

In this diagram, the single electron is assigned to the 1s sublevel, which is the lowest-energy sublevel in the lowest-energy level. For helium (atomic number 2), which has two electrons, the electron configuration is 1s2 and can be represented by the following box diagram:

The element lithium (atomic number 3) has three electrons and its electron configuration is 1s22s1. This can be represented by the following box diagram:

The element boron (atomic number 5) has five electrons and its electron configuration is 1s22s22p1. This can be represented by the following box diagram:

Table 5.2 shows the electron configurations of the elements with atomic numbers 1 through 18.
In conclusion, the element with 17 protons, 18 neutrons, and 18 electrons is chlorine. Chlorine has an atomic number of 17 and an atomic weight of 35.453 g/mol. Isotopes of chlorine have different numbers of neutrons, with the common isotope having an atomic weight of 35 amu and the rare isotope having an atomic weight of 37 amu. The electron configuration of chlorine is 1s22s22p63s23p5, which can be represented by box diagrams.
What element has 17 protons 20 neutrons and 17 electrons?
Atoms are made up of protons, neutrons, and electrons and these different components determine the element’s identity and characteristics. The element with 17 protons, 20 neutrons, and 17 electrons is chlorine.
Chlorine is an element found in Group 17 of the periodic table. It is a halogen, meaning that it is a highly reactive non-metal. Chlorine is a pale yellow-green gas at room temperature, but it is found in nature as a yellow-green liquid or solid.
Isotopes
Although every atom of an element has the same number of protons, the number of neutrons can vary. This creates different versions of the same element, called isotopes.
For example, all chlorine atoms have 17 protons and 17 electrons, but three out of four chlorine atoms weigh 35 amu (17 protons and 18 neutrons) and the fourth weighs 37 amu (17 protons and 20 neutrons). These are the isotopes of chlorine. The atomic weight reported in the periodic table is the average weight of 35.5 amu.
Ions
Ions are atoms that have gained or lost electrons. All atoms of an element have the same number of protons, but they may differ in the number of neutrons they have.
For example, four helium isotopes are shown in Figure 1-1. All atoms of chlorine have 17 protons, but there are chlorine isotopes with 15 to 23 neutrons. Only two chlorine isotopes exist in significant amounts in nature, those with 18 neutrons (75.53% of all chlorine atoms found in nature), and those with 20 neutrons (24.47%).
To write the symbol for an isotope, place the atomic number as a subscript and the mass number (protons plus neutrons) as a superscript to the left of the atomic symbol. The symbols for the two naturally occurring isotopes of chlorine then would be Cl-35 and Cl-37. In discussing these isotopes, we use the terms chlorine-35 and chlorine-37.
Composition of Typical Atoms and Ions
Table 1-2 shows the composition of typical atoms and ions. For a nucleus to be stable, the number of neutrons should (for the first few elements) equal or slightly exceed the number of protons.
Example 1.2.2
Calculate the mass that is lost when an atom of carbon-12 is formed from protons, electrons, and neutrons.
Solution: The chlorine isotope has 17 protons and 20 neutrons:
Protons: 17 X 1.00728 amu = 17.1238 amu
Neutrons: 20 X 1.00867 amu = 20.1734 amu
Electrons: 17 X 0.00055 amu = 0.0094 amu
Total particle mass: 37.3066 amu
Actual observed atomic weight: 36.966 amu
Mass Loss: 0.341 amu
Each isotope of an element is characterized by an atomic number (total number of protons), a mass number (total number of protons and neutrons), and an atomic weight (mass of atom in atomic mass units). If there are several isotopes of an element in nature, then of course the experimentally observed atomic weight (the natural atomic weight) will be the weighted average of the isotope weights. The average is weighted according to the percent abundance of the isotopes.
Therefore, the element with 17 protons, 20 neutrons, and 17 electrons is chlorine, with the isotope symbols Cl-35 and Cl-37. Chlorine is an element found in Group 17 of the periodic table and it is a highly reactive non-metal.


Leave a Comment