Difference Between Constitutional Isomers and Resonance Structures
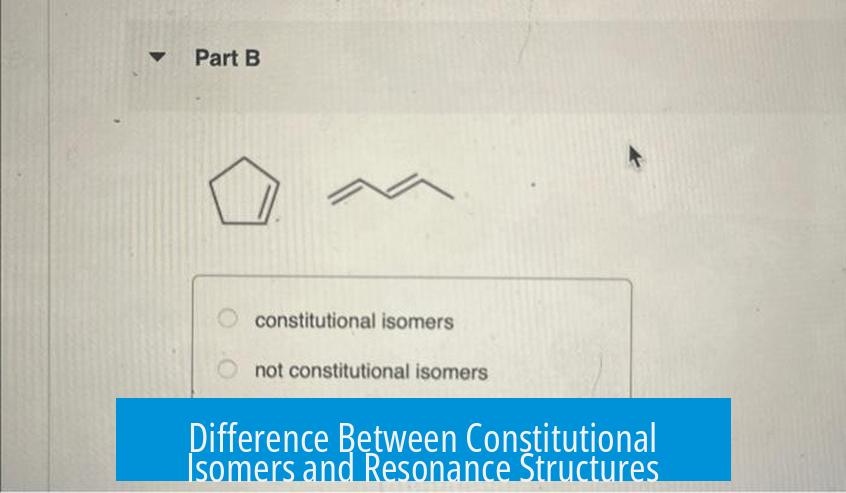
Constitutional isomers and resonance structures are not the same. Constitutional isomers are distinct molecules differing in atom connectivity, while resonance structures are different electron arrangements within the same molecule.
What Are Resonance Structures?
Resonance structures are multiple Lewis structures depicting the same molecule. They illustrate different arrangements of electrons without altering atoms or bonds. The core skeleton remains fixed, while electrons shift positions.
- Show electron delocalization.
- No change in atom connectivity.
- Represent a single molecular entity.
What Are Constitutional Isomers?
Constitutional isomers are separate compounds with the same molecular formula but different atom connections. This difference changes physical and chemical properties. Examples include pentane and 2-methylbutane.
- Atoms connect differently.
- Distinct molecules with unique names.
- Involve rearrangement of the atomic skeleton.
Key Differences: Atom vs Electron Movement
| Aspect | Constitutional Isomers | Resonance Structures |
|---|---|---|
| Molecular Identity | Different compounds | Same molecule |
| Atom Connectivity | Different | Identical |
| Electron Arrangement | Same (within molecule) | Different (electrons only) |
| Atom Movement | Yes | No |
Summary

- Resonance structures show electrons moving within unchanged atomic framework.
- Constitutional isomers involve atoms bonded differently, creating distinct molecules.
- Resonance structures explain electron delocalization.
- Constitutional isomers demonstrate how connectivity affects molecular identity.
What’s the Difference Between Constitutional Isomers and Resonance Structures? Are They the Same Thing?
If you’re wondering whether constitutional isomers and resonance structures are the same, the quick answer is: no, they’re not the same thing. While both concepts deal with molecules and atoms, they involve very different processes and outcomes. Let’s unravel this chemistry riddle with clarity, a sprinkle of humor, and some real-world examples.
Ready? Let’s dive in!
Resonance Structures: A Peek at Electron Dance Moves
Resonance structures are not different molecules but different ways of drawing the same molecule. Think of them as snapshots capturing electrons showing off their dance moves within the molecule’s framework. Electrons shift positions, but the atoms stay put.
To put it simply, resonance structures are multiple Lewis structures for one single molecule. They visually represent how electrons can move around, delocalizing over bonds without altering the molecule’s backbone. Imagine electrons as party guests who like to circulate, but the house itself—the atoms and how they’re connected—doesn’t change.
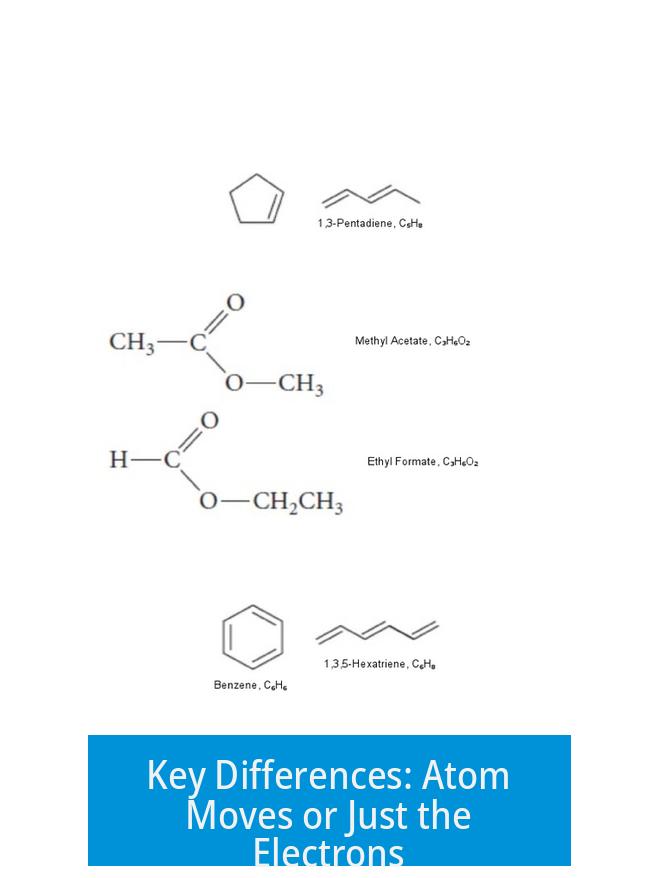
This electron relocation explains why some molecules exhibit stability beyond what a single structure could suggest. Classic examples? Benzene’s aromatic ring, where multiple resonance structures contribute to overall stability and unique properties.
Constitutional Isomers: When Atoms Switch Places
Constitutional isomers are a whole different story. Here, atoms themselves change their connections. These are different molecules that share the same formula but have distinct arrangements of atoms. It’s like rearranging the furniture in your house into a new layout. Same furniture, different room arrangement.
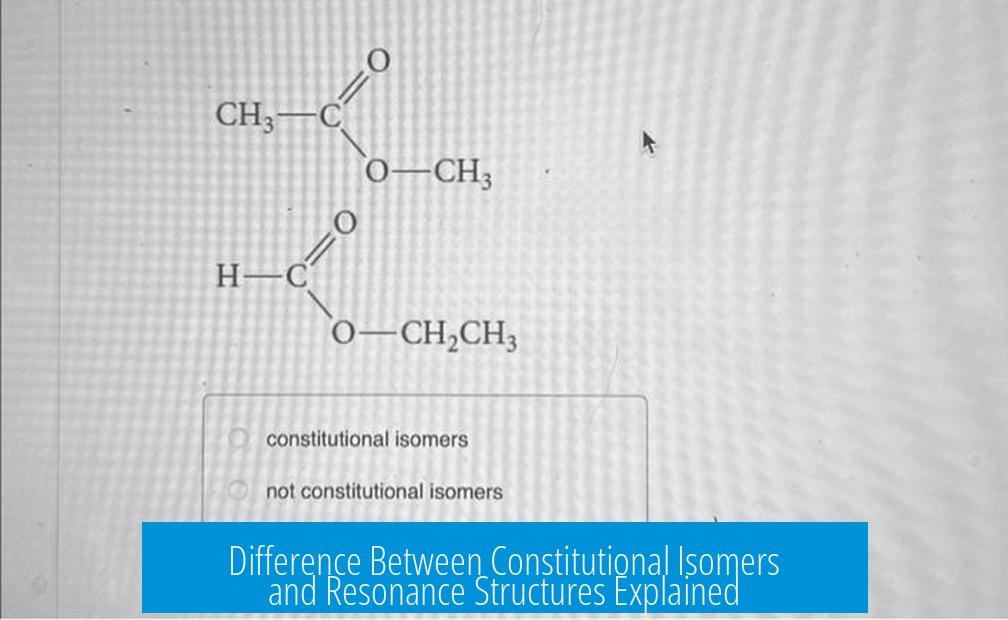
For instance, pentane and 2-methylbutane both have the formula C5H12, but their atoms are bonded differently, resulting in distinct structures and properties. They might share a formula, but their identities differ—they get different names, behave differently in reactions, and pack distinct physical responses.
Key Differences: Atom Moves or Just the Electrons?
| Aspect | Resonance Structures | Constitutional Isomers |
|---|---|---|
| Are they the same molecule? | Yes, just different electron arrangements. | No, different molecules with distinct atom connectivity. |
| Do atoms move or change connectivity? | No movement of atoms, only electrons shift. | Yes, atoms change bonding partners. |
| Do they have different names? | No, same molecule. | Yes, different compounds have different names. |
| Example | Benzene resonance forms | Pentane vs. 2-methylbutane |
Why Does This Difference Matter?
If you’re a chemistry student or professional, confusing resonance structures with constitutional isomers can lead to misconceptions in understanding molecular behavior.
Resonance explains molecular stability by showing electron delocalization. Ignoring resonance may make molecules seem unstable or reactive when they’re actually quite steady. On the other hand, confusing constitutional isomers with one molecule would mess up your chemical reactions’ predictions since structure dictates function and reactivity.
Understanding this distinction also helps in drawing accurate molecular diagrams, predicting reaction mechanisms, and interpreting spectra.
Practical Tip: How to Tell If You’re Looking at Resonance Structures or Constitutional Isomers?
- Check the atoms: If the atoms are connected differently, you’re dealing with constitutional isomers.
- Check the bonds: In resonance, bonds don’t break or form differently; only electron positions shift within the same bonds.
- Name game: Different names? Probably constitutional isomers.
For example, resonance structures of the nitrate ion (NO3-) all have the same connectivity but different placements of double bonds and charges. In contrast, isomers like ethanol and dimethyl ether share a formula (C2H6O) but connect atoms differently and bear unique names and properties.
To Sum Up: They’re Totally Not the Same, Yo
Resonance structures are all about electrons moving inside the lock, no rearranging of the key itself. Constitutional isomers are about swapping out keys for different ones altogether—even if they fit a similar lock (molecular formula).
So next time you see those clever line drawings with shifting electrons, remember they’re just different views of the same molecular dance. But if atoms change their partnerships? That’s a whole new molecule stepping onto the stage.
Want to explore more?
Dive into resources on molecular orbital theory and isomer classification for deeper insights. And if you’re feeling adventurous, try drawing resonance structures and isomers for simple compounds like butadiene, or cyclohexane derivatives—nothing beats practice to solidify concepts.
Got questions or fun chemistry puzzles? Let’s chat—because understanding the subtle yet crucial differences between these concepts sharpens your scientific thinking and impresses your lab partners. Science, after all, loves clarity as much as curiosity.




Leave a Comment