Why We Do Not Add “Benzene” in Naming When a Benzene Ring Is Present
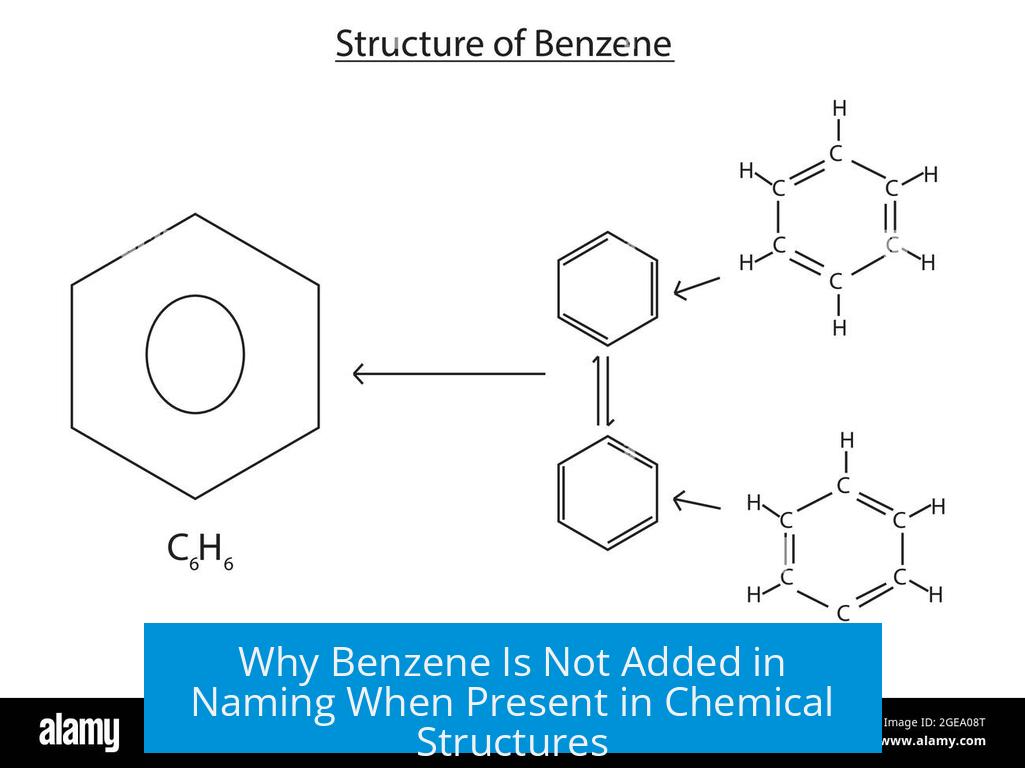
Benzene is not explicitly added in naming when it forms the main ring because it acts as the parent structure, not a functional group or substituent.
Benzene as Parent Compound
When benzene forms the central ring in a molecule, it serves as the core structure. In such cases, the compound is named as a benzene derivative. The benzene ring is not considered an add-on functional group but the base framework.
Only when benzene appears as a substituent—such as phenyl (-C6H5) or benzyl (-CH2-C6H5)—is it named explicitly as a substituent. This distinction prevents redundant naming.
Benzene Is Not a Functional Group
Official nomenclature does not classify benzene itself as a functional group. It is a distinct molecule (C6H6) and part of the molecule’s backbone rather than a group modifying the parent structure.
The term arene is used more broadly to describe aromatic hydrocarbons, representing the functional group type of molecules with benzene-like rings.
Aromatic Ring as Backbone vs. Substituent
The aromatic ring in benzene-containing molecules functions as a backbone structure. It is the ring system to which other groups may attach.
- If benzene is the main ring, it forms the parent compound.
- If a benzene unit is attached as a side chain, it is labeled as phenyl or benzyl.
Functional Groups Involving Aromatic Rings
Some aromatic compounds include functional groups such as alcohols (phenol) on the benzene ring. Here, the phenol group is named to reflect both the arene and the functional group (alcohol).
However, the benzene ring alone, without attached functional groups, does not qualify as a functional group that requires naming.
Summary of Key Points
- Benzene serves as the parent ring in aromatic compounds rather than a functional group or substituent.
- Phenyl and benzyl names apply only when benzene appears as a substituent outside the main ring.
- Benzene is a specific molecule, while arenes define the broader functional group class of aromatic rings.
- Functional groups attached to benzene, such as hydroxyl in phenol, are named, but benzene itself is treated as the backbone.
Why don’t we add “benzene” to the name when there is a benzene ring in the molecule?
Benzene serves as the parent compound when it forms the main ring. It is not listed separately because it is the core structure, not a substituent. Naming focuses on substituents outside the benzene ring.
Is benzene considered a functional group in chemical nomenclature?
No, benzene is not officially categorized as a functional group. It is viewed as part of the molecule’s backbone rather than a functional substituent.
When do we label phenyl or benzyl groups instead of benzene?
We name phenyl or benzyl groups only when they act as substituents attached to a different parent chain or ring. If benzene is the main ring, it remains the parent and is not named as a substituent.
What is the difference between benzene, phenyl, and arenes in terms of naming?
Benzene is a specific molecule (C₆H₆). Phenyl is a substituent derived from benzene. “Arene” is the general functional group term for aromatic hydrocarbons that include benzene rings.
Can benzene combined with other groups form named functional groups?
Yes. For example, phenol contains a benzene ring with an –OH group and is named as a functional group. Benzene alone, however, is not considered a functional group.



Leave a Comment