Why Does the Nitrile Group Attach to the Carbonyl Without the Nitro Group?
The nitrile group attaches preferentially to the carbonyl without the nitro substituent because the irreversible step after benzaldehyde attack favors product formation, while reversible intermediates from the nitro-substituted carbonyl and unfavorable cyanide elimination limit attachment there.
Reaction Pathway Preference
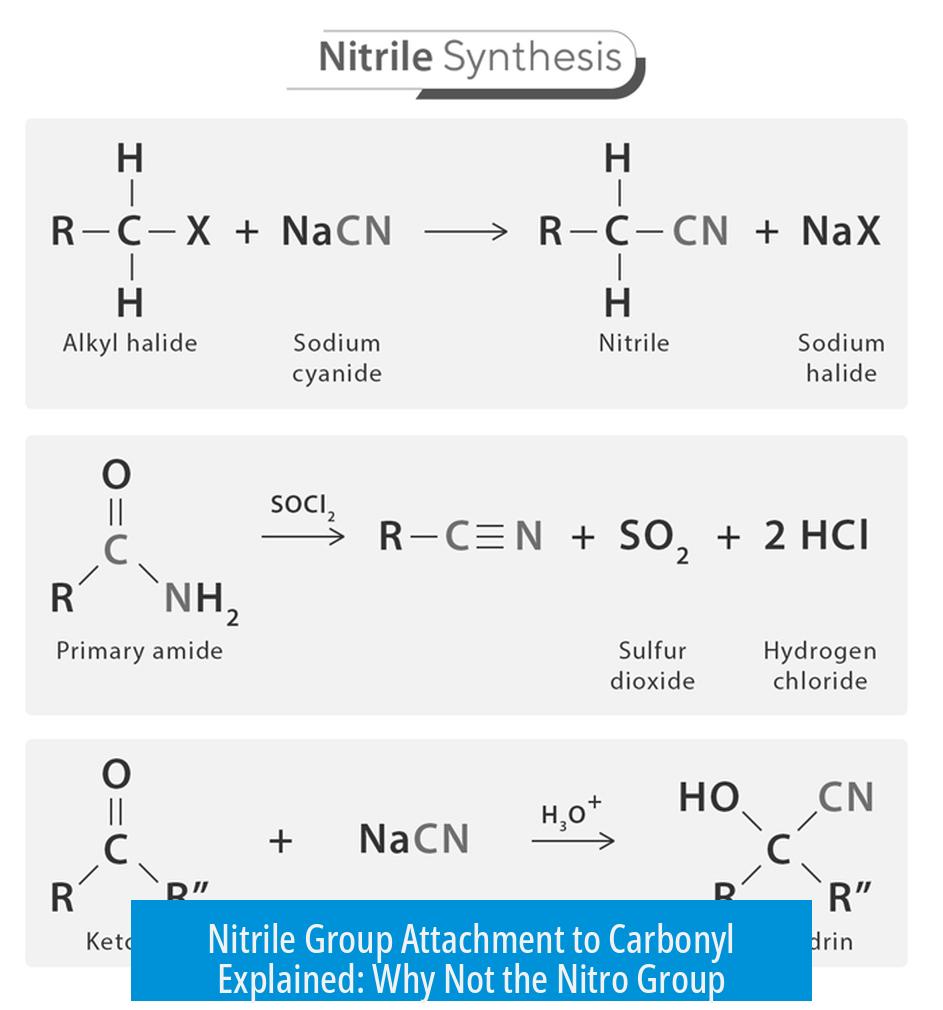
The nitrile nucleophile may attack either the carbonyl on nitrobenzaldehyde or benzaldehyde. However, the tetrahedral intermediate formed by attack on nitrobenzaldehyde is reversible. This reversibility means no stable product accumulates from this path. Conversely, attack on benzaldehyde leads to an irreversible step, driving the reaction forward. Thus, the productive attachment favors the non-nitro carbonyl.
Electronic Effects of the Nitro Group
The nitro group exerts a strong electron-withdrawing effect, making the aromatic ring and the carbonyl carbon more electrophilic. This effect theoretically enhances carbonyl reactivity towards nucleophiles like nitriles. Despite this, the nitrile attaches less often to the nitro-substituted carbonyl, indicating other mechanistic factors dominate.
Impact of Cyanide Elimination on Equilibrium
The reaction involves a key cyanide elimination step before final product formation. When the cyanide is attached to the nitro-substituted carbonyl, elimination becomes less favorable. This diminution shifts the reaction equilibrium backward, disfavoring stable product formation at this site.
Experimental Reality and Product Mixtures

In practice, the reaction may produce a mixture of products, including minor amounts where nitrile attaches to the nitro-substituted carbonyl. These minor products often go unreported, emphasizing that the main observed product features nitrile attachment at the carbonyl lacking the nitro group.
Role of Inductive Effects on Carbonyl Reactivity
The nitro group’s inductive effect increases carbonyl electrophilicity, strengthening nucleophilic attraction. This factor alone does not govern the nitrile’s attachment choice. Instead, the combination of reversibility, elimination steps, and reaction kinetics determine the outcome more decisively.
Key Points
- Nitrile attacks both carbonyls but irreversible formation favors benzaldehyde-derived product.
- Reversible intermediates with nitro-substituted carbonyl reduce productive attachment.
- Cyanide elimination is less favorable at the nitro-carbonyl, affecting equilibrium.
- Electron-withdrawing nitro increases electrophilicity but does not override kinetic and thermodynamic controls.
- Minor products from nitro-substituted carbonyl attack exist but are usually omitted in reports.
Why Does the Nitrile Group Attach to the Carbonyl Without the Nitro Group?
So, you’re wondering why the nitrile group prefers to cozy up to a carbonyl group that’s *not* sporting a flashy nitro buddy? The short, strong answer is this: the nitrile group attaches to the carbonyl without the nitro group because the reaction pathway involving the non-nitro benzaldehyde is the one with an irreversible and therefore productive step, while attack on the nitro-substituted carbonyl remains reversible and less favorable overall.
Let’s unwrap this chemical mystery layer by layer, peppered with some facts, practical insights, and even a sprinkle of chemistry drama. Ready to dive in?
1. The Reaction Pathway Preference: A Tale of Two Carbonyls

Picture the nitrile group at a crowded party with two potential dance partners: a carbonyl on non-nitro benzaldehyde and one on nitrobenzaldehyde. Initially, the nitrile might be flirtatious with both, but it quickly finds the nitro-substituted carbonyl more attractive due to its electronic vibes.
Why? The nitro group makes the benzene ring electron-deficient, which in theory pulls electrons out of the carbonyl, rendering it a better electrophile—essentially sparkling brighter on the dance floor.
Despite this initial preference, the nitrile’s attachment to the nitro-substituted carbonyl forms a tetrahedral intermediate that’s reversible. This reversibility means the nitrile can easily break up and move on, making no lasting bond.
On the other hand, when the nitrile attacks the carbonyl without the nitro group, the reaction proceeds through a pathway with an irreversible step. This permanence seals the deal, producing the final product and pulling the reaction forward.
2. The Electronic Enigma: Nitro Group’s Electron Game
This brings us to the nitro group’s role—not as a matchmaker for the nitrile, but as a meddler of electrons.
| Effect | On Benzaldehyde Without Nitro | On Nitrobenzaldehyde |
|---|---|---|
| Electron Density | Normal electron density on carbonyl | Electron-deficient aromatic ring, draining electron density from carbonyl |
| Electrophilicity | Moderate electrophilicity | Enhanced electrophilicity due to nitro group |
At first glance, you’d think making the carbonyl more electrophilic (by way of the nitro group’s electron withdrawal) would make it the star attraction for nucleophiles. But chemistry isn’t just a popularity contest based on electronegativity!
3. Cyanide Elimination: The Plot Twist That Shifts Equilibrium
The penultimate step of the reaction often involves losing cyanide (a bit like a dramatic exit scene). Now, here’s a subtle but effective twist: if the cyanide was attached to the nitro-substituted carbonyl, this elimination step becomes less favorable.
Why does this matter? Because less favorable elimination nudges the reaction equilibrium backwards. The chemical storyline then discourages forming products with the nitrile group attached to the nitro carbonyl because the reaction can’t “close the deal” easily.
This sets up a chemical bias, funneling the reaction down the pathway involving the carbonyl without the nitro group.
4. The Experimental Reality Check: Mixtures Galore
Hold on though—real-life reactions rarely stick to a single neat storyline. Despite theoretical arguments, the actual reaction often produces a mixture of products. It’s like baking cookies intending chocolate chip but ending up with a batch containing some oatmeal raisin. Chemistry has its quirks.
Experimental work sometimes omits minor product pathways for clarity or feasibility. But chemists in the know understand that mixtures arise and conditions (like solvents, temperature, catalysts) can tilt the balance.
5. Inductive Effects: The Subtle Influence Behind the Scenes

Another contributing factor? The inductive effect of the nitro group. It exerts a strong electron-withdrawing influence via sigma bonds, pulling electron density away from the carbonyl carbon. This affects how electrophilic (electron-loving) the carbonyl becomes—technically increasing electrophilicity but also influencing how intermediates stabilize.
It’s a bit like a magnet affecting iron filings nearby: not always immediately visible, but impactful all the same.
Summing it All Up: What’s Really Going On?
The nitrile group’s preference for attaching to the carbonyl without the nitro group stems from a delicate dance of reaction kinetics and thermodynamics:
- Reversible vs. Irreversible Steps: Attack on the nitro carbonyl forms reversible intermediates; the carbonyl without nitro sees irreversible product formation.
- Cyanide Elimination: Less favorable when nitrile bonds to nitro-substituted carbonyl, pushing the equilibrium away from such products.
- Electronic Effects: Nitro’s electron withdrawal changes carbonyl properties but also stabilizes intermediate differently, affecting the reaction outcome.
- Real-World Complexity: Mixtures of products likely form, but major products arise from the most favorable pathway.
Understanding these factors empowers chemists to manipulate reaction conditions or substrates to steer outcomes as desired. It’s like choosing the right ingredients, oven temperature, and baking time to get that perfect cookie instead of a confused batch.
Practical Tips for Chemists and Enthusiasts
- Consider Reaction Conditions: Altering solvent polarity, temperature, or catalyst can shift the balance between possible products.
- Use Computational Tools: Modeling reaction pathways can help predict which intermediates are stable and which steps are irreversible.
- Careful Product Analysis: Employ techniques like NMR or mass spectrometry to detect minor products sometimes overlooked.
- Design Substrates Thoughtfully: Introducing or removing electron-withdrawing groups can drastically affect reaction selectivity.
Final Thoughts
Why does the nitrile group shy away from the nitro carbonyl despite its initial allure? Because chemical reactions are about the *whole* journey, not just the first handshake. Irreversible steps, elimination favorability, and electronic influences write the script for where the nitrile finally settles down.
Next time you look at a reaction mechanism, remember: the chemistry story is never just about who looks more electrophilic. It’s about who gets to stay on stage when the curtains close.




Leave a Comment