Why Would This Reaction Proceed via SN1 Despite Using DMSO and Strong Nucleophiles?
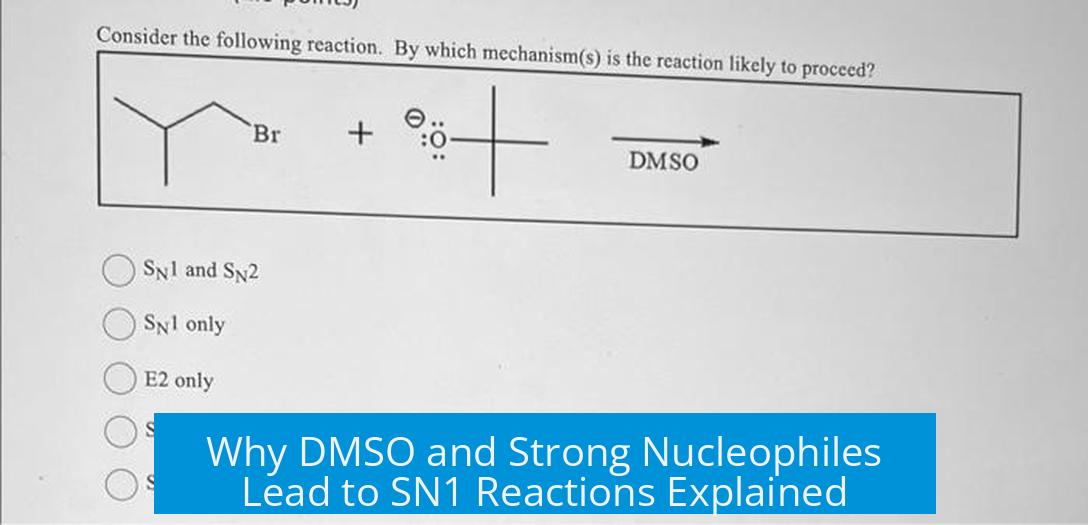
This reaction proceeds via an SN1 mechanism because the substrate contains an excellent leaving group (tosylate, Tos) and the nucleophile, methanol (CH3OH), is weak and neutral, despite the solvent being DMSO, an aprotic solvent capable of supporting strong nucleophiles.
Understanding the Role of the Nucleophile
Although hydroxide ion (OH−) is a strong nucleophile due to its ionic nature (Na+OH−), methanol (CH3OH) is neutral and much less nucleophilic. In SN1 reactions, the nucleophile generally does not affect the rate, because the rate-determining step involves the formation of a carbocation intermediate after the leaving group departs.
The neutral CH3OH does not favor a concerted SN2 pathway, especially if the carbocation intermediate is stabilized. Hence, even in an aprotic solvent like DMSO, which can support nucleophilic species, the reaction can prefer SN1 if the nucleophile is weak.
Solvent Effects and Mechanism Choice
- DMSO is an aprotic solvent, which usually favors SN2 mechanisms by stabilizing cations and not solvating nucleophiles strongly.
- However, solvent effects are overshadowed here by the substrate’s nature and nucleophile strength.
- The substrate’s leaving group is tosylate (Tos), an excellent leaving group that easily departs to form a carbocation.
Significance of Tosylate (Tos) as a Leaving Group
Tosylates are resonance-stabilized and negatively charged leaving groups, making them very good at leaving. This fosters carbocation formation, which drives the reaction toward SN1 rather than SN2.
Summary table of key factors
| Factor | Effect on Mechanism |
|---|---|
| Nucleophile | CH3OH is weak, favors SN1; OH− would favor SN2 but is absent here |
| Solvent (DMSO) | Aprotic; generally supports SN2 but does not dominate over substrate effects |
| Leaving Group (Tosylate) | Excellent leaving group, favors carbocation formation and SN1 |
Key Takeaways
- CH3OH is neutral and weakly nucleophilic; this disallows a strong SN2 pathway.
- Tosylate is a superb leaving group that facilitates carbocation formation.
- DMSO as an aprotic solvent supports SN2 but substrate and nucleophile strengths are more decisive.
- Hence, the reaction proceeds via SN1 because the rate-limiting carbocation formation dominates.



Leave a Comment