Yield vs Yield Based on Recovered Starting Material
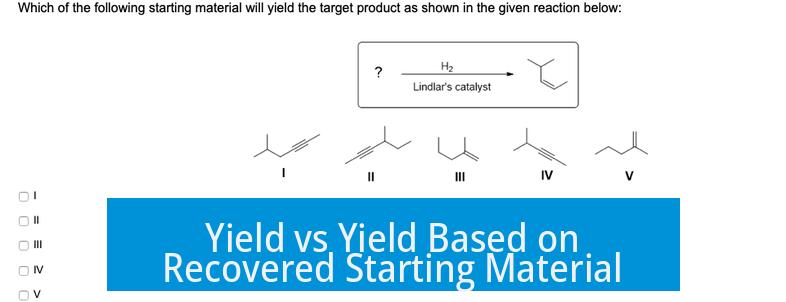
Yield based on recovered starting material (BRSM) and yield based on initial starting material differ fundamentally in how they interpret reaction efficiency. Yield based on initial starting material measures product relative to all material introduced. BRSM calculates yield relative only to the material actually consumed in the reaction.
Definitions and Concepts of Yield Calculations
In a reaction starting with 100 mmol of starting material (SM), suppose 7.3 mmol of product forms, and 90 mmol of SM remains unreacted. The yield relative to material consumed (which is 10 mmol) is 7.3/10 = 73%. This calculation excludes unreacted SM and focuses on how efficiently consumed material converts to product.
Conversely, overall yield based on the initial 100 mmol is 7.3/100 = 7.3%. This figure reflects total efficiency including unreacted material, providing a realistic measure of the entire process.
Practical Implications in Reporting
BRSM often portrays reactions more favorably by ignoring unreacted starting material. A high BRSM yield can mask low overall conversion. For instance, reporting a 73% BRSM yield sounds better than the true 7.3% overall yield.
Chemists may prefer BRSM in publications to emphasize successful parts of reactions. However, this practice requires transparency to avoid misinterpretation.
Analytical Considerations and Challenges
- BRSM yield assumes the starting material consumed fully converted to product or byproducts, which might not always be true.
- If a large fraction of SM disappears without forming desired product, side reactions or decomposition may reduce actual efficiency.
- Overall yield offers a more conservative but honest metric reflecting full material use.
Critical Perspectives
Viewing BRSM yield as “polishing a turd” highlights skepticism. High BRSM may obscure poor reaction performance seen when accounting for all starting material.
Ultimately, both yields provide information. Reporting both allows clearer understanding of reaction efficiency and helps avoid overestimation.
Key Takeaways
- Yield based on recovered starting material (BRSM) measures product formed relative to consumed SM.
- Overall yield relates product to total SM initially introduced.
- BRSM can make low-conversion reactions look more efficient.
- Overall yield gives realistic view of reaction productivity.
- Transparent reporting of both values improves clarity in chemical research.
What does yield based on recovered starting material (BRSM) mean?
BRSM calculates yield using the amount of starting material actually consumed, not the total amount initially added. This shows how much product forms from the reacted portion only.
How does overall yield differ from BRSM yield?
Overall yield compares product amount to initial starting material added. It accounts for all material regardless of how much reacted, often showing a lower yield than BRSM.
Why might BRSM yield be reported instead of overall yield?
BRSM can make a reaction appear more efficient if a large amount of starting material was recovered. This can improve reported numbers but may mask poor conversion or product formation.
What issues can arise when yield based on consumed material and actual product differ greatly?
If much starting material disappears but little product appears, it suggests side reactions or losses. Yield based only on consumed starting material may overestimate reaction success.
Is it valid to use BRSM yield as the sole measure of reaction efficiency?
BRSM yield can be misleading alone. It’s important to consider both overall yield and product purity to get a true picture of reaction performance.





Leave a Comment