Atomic mass is an incredibly important concept to understand when it comes to the periodic table of elements. Knowing the atomic mass of each element can help us understand the structure and behavior of matter, and can even be used to determine the elemental and atomic composition of a sample of matter. But for those who are just beginning to learn about the periodic table, the concept of atomic mass can be daunting.
So, how do you find the atomic mass of a beginner? In this article, we’ll explain the basics of atomic mass, the different ways to calculate it, and provide some tips and tricks for understanding atomic mass.
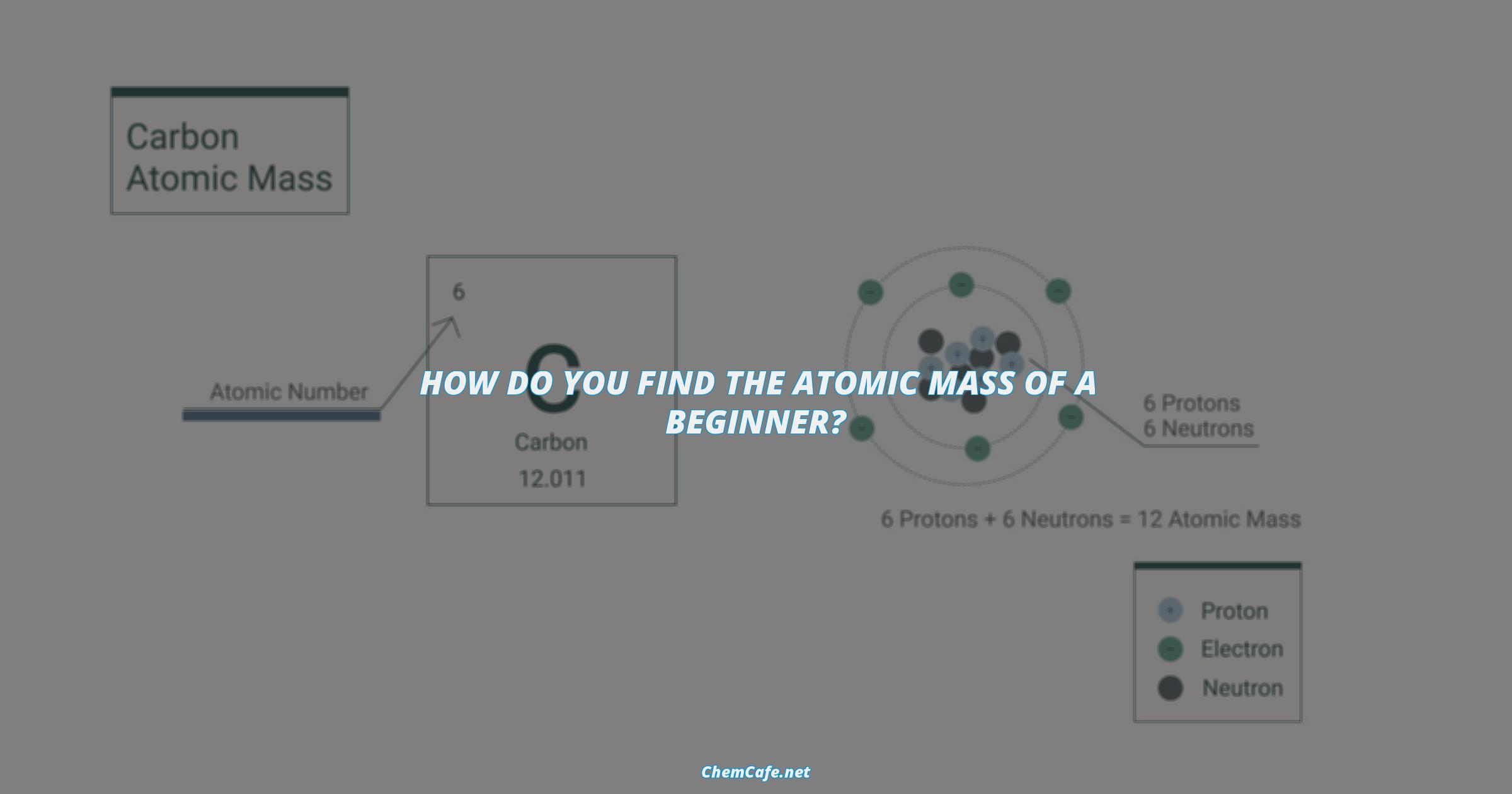
Atomic mass is simply the number of protons plus the number of neutrons in an atom when looking at a periodic table. This is typically calculated by adding the number of protons and neutrons together, as the electrons are too small to be considered. The importance of atomic mass is highlighted by the invention and use of the mass spectrometer, a technological system used to find the elemental and atomic composition of a sample of matter.
The mass number of an atom of a specific element can be determined by using the formula Mass Number = Number of Protons + Number of Neutrons. Daltons, which are the standard units used for measuring atomic mass, are equal to Atomic Mass Units (amu).
Knowing the atomic mass of an element can be incredibly useful. But, if you’re a beginner, it can be difficult to figure out. Thankfully, there are three ways to determine the atomic mass, depending on the given conditions.
The first way is to simply look up the atomic mass on the periodic table. This can be done by looking at the digit marked under the element’s representation. The second option is to use the mass spectrometer, which uses the principle of mass-to-charge ratio to identify the elemental and atomic composition of a sample of matter.
The third way to calculate atomic mass is to use the atomic mass trick. This involves using the atomic mass of two adjacent elements on the periodic table, and subtracting their atomic masses from each other. This will give you the atomic mass of the element in question.
By understanding the basics of atomic mass, and being aware of the different ways to calculate it, you can easily learn how to find the atomic mass of a beginner. With this knowledge, you’ll be well on your way to becoming a periodic table expert!
How do you find the atomic mass of a beginner?
Atomic mass is an important concept to understand when learning about the different elements in the periodic table. Mass number is simply the number of protons plus the number of neutrons in an atom when looking at a periodic table. The importance of atomic mass is highlighted by the invention and use of the mass spectrometer.
What is a Mass Spectrometer?
The mass spectrometer is a technological system used to find the elemental and atomic composition of a sample of matter in question. This process involves the use of a beam of particles, such as electrons or ions, that are accelerated and then passed through a magnetic field. Upon passing through the magnetic field, the particles are deflected and their mass is measured based on the amount of deflection.
What is the Atomic Mass?
The atomic mass or atomic weight is the average number of protons and neutrons in an element for all of its isotopes. This number can be found on the periodic table of elements and is typically expressed in atomic mass units (amu). One atomic mass unit is equal to one-twelfth of the mass of a single carbon-12 atom.
How to calculate the Atomic Mass?
Here are three ways to determine the Atomic Mass, depending on the given conditions:
By looking up to the atomic mass on the periodic table– In the periodic table digit of an atomic mass is usually marked under the representation of an element. For instance, if you are looking for the atomic mass of oxygen, you will find the number 8.00 on the periodic table. This means that the oxygen atom has an atomic mass of 8.00 amu.
By using the formula to calculate the mass number– The formula below can be used to determine the mass number of an atom of a specific element when looking at the periodic table of elements:
Mass Number = Number of Protons + Number of Neutrons
For example, if you need to find the mass number of an atom of sulfur, you can look up the periodic table and find that sulfur has 16 protons and 18 neutrons. By applying the formula, you will get a mass number of 34.
By using a mass spectrometer– The mass spectrometer is the most accurate way to determine the atomic mass of an element. This device measures the mass of an atom or molecule and displays it as a graph. By analyzing the graph, the atomic mass of a sample can be determined.
Atomic mass is an important concept to understand when studying the periodic table. The mass number of an atom is the sum of its protons and neutrons, and the atomic mass is the average mass of an element’s isotopes. Atomic mass can be determined by looking up the periodic table, using the formula to calculate the mass number, or by using a mass spectrometer.
How do you find the mass of an atom?
Atoms are the building blocks of all matter and understanding the mass of an atom is essential for studying the properties of matter.
Atomic mass is the sum of the masses of the protons, neutrons, and electrons in an atom, or the average mass, in a group of atoms. Knowing the atomic mass of an element can help you determine the ratio of its isotopes, the abundance of its isotopes in a sample, and the density of a substance.
What Is Atomic Mass?
Atomic mass is the total mass of an atom which is determined by the number of protons and neutrons it contains. The mass of a single atom is extremely small, so it is usually expressed in atomic mass units (amu). One atomic mass unit (amu) is equal to 1.66 x 10-24 grams.
Atomic mass is also referred to as the “atomic weight” of an atom. This term is used to describe the average mass of an element, taking into account the different isotopes that make up the element.
Look Up Atomic Mass on the Periodic Table
The periodic table is a useful tool for finding the atomic mass of an element. The atomic mass of each element is listed on the periodic table. For example, the atomic mass of oxygen is 16.00 amu and the atomic mass of carbon is 12.01 amu.
The atomic mass of an element can also be determined by calculating the average of its isotopes. Isotopes are atoms of the same element that have different numbers of neutrons. An element can have several different isotopes, each with its own unique atomic mass.
Finding the Averaged Atomic Weight of an Element
To find the averaged atomic weight of an element, you must first determine the abundance of each isotope in a sample. This can be done by measuring the mass of the sample and dividing it by the atomic mass of each isotope.
Once you have determined the abundance of each isotope, you can calculate the averaged atomic weight of the element by multiplying the abundance of each isotope by its atomic mass and then adding the results together.
Calculating Atomic Mass from a Known Ratio of Isotopes
If you know the ratio of isotopes in a sample, you can calculate the atomic mass of the element. This is done by multiplying the atomic mass of each isotope by its abundance in the sample and then adding the results together.
For example, if you have a sample of oxygen that consists of 99.76% oxygen-16 and 0.04% oxygen-18, you can calculate the atomic mass of oxygen by multiplying 16.00 (the atomic mass of oxygen-16) by 0.9976 (the abundance of oxygen-16) and 0.04 (the abundance of oxygen-18) by 18.00 (the atomic mass of oxygen-18). This will give you an atomic mass of 15.999 amu.
The mass of an atom is a weighted average that is largely determined by the number of its protons and neutrons, whereas the number of protons and electrons determines its charge. Each atom of an element contains the same number of protons, known as the atomic number (Z).
The method used to find atomic mass depends on whether you’re looking at a single atom, a natural sample, or a sample containing a known ratio of isotopes. In most cases, you can look up the atomic mass on the periodic table. If you need to calculate it from a known ratio of isotopes, you can use the formula described above.
How do we calculate atomic weight?
Atomic weight is a measure of the average mass of an element’s atoms and is expressed in atomic mass units (amu, u, or Da). It is an important factor in determining the properties of a chemical, so it is important to understand how to calculate atomic weight.
What is Atomic Weight?
Atomic weight is the sum of the masses of each isotope of an element multiplied by its fractional abundance. Isotopes are atoms of the same element that have different atomic mass numbers (protons + neutrons). For example, carbon has three isotopes: carbon-12, carbon-13, and carbon-14. The fractional abundance of each isotope is calculated by dividing the number of atoms of each isotope by the total number of atoms of all isotopes.
How Is Atomic Weight Calculated?
To calculate the atomic weight of an element, the mass of each isotope must be known and the fractional abundance of each isotope must be calculated. The atomic weight is then calculated by adding the mass of each isotope multiplied by its fractional abundance.
For example, if the mass of carbon-12 is 12.00u and the fractional abundance of carbon-12 is 98.89%, the atomic weight of carbon can be calculated as follows:
Atomic Weight of Carbon = (12.00u x 0.9889) + (13.00u x 0.0111) = 12.01u
What is the Standard Unit of Atomic Mass?
The original standard of atomic weight, established in the 19th century, was hydrogen, with a value of 1. From about 1900 until 1961, oxygen was used as the reference standard, with an assigned value of 16. The unit of atomic mass was thereby defined as 1/16 the mass of an oxygen atom. In 1961, the standard unit of atomic mass was changed to one-twelfth the mass of an atom of the isotope carbon-12.
Atomic weight is an important factor in determining the properties of a chemical element. It is calculated by adding the mass of each isotope multiplied by its fractional abundance. The standard unit of atomic mass is one-twelfth the mass of an atom of the isotope carbon-12. The calculated worth of the material depends on the atomic weights used in the calculations. Knowing how to calculate atomic weight is important for accurately determining the properties of a chemical element.
How do you find the atomic mass trick?
When it comes to understanding the atomic masses of elements, it can be difficult to figure out the correct answer. This is where the atomic mass trick comes in. It is a helpful tool that chemists use to accurately calculate the atomic masses of different elements.
The atomic mass trick is based on the fact that the mass of an atom is equal to the sum of the masses of its protons, neutrons, and electrons. This means that the atomic mass of any element can be determined by adding together the masses of its protons, neutrons, and electrons.
Relative Atomic Mass
The link between the mass of an element and the number of atoms it contains is the relative atomic mass of the element. By using this, chemists can work out the chemical formula of a substance. The relative atomic mass scale is used to compare the masses of different atoms. This means that one atom is given a relative atomic mass of exactly 12.
How to Calculate Atomic Mass?
There are three ways to calculate the atomic mass, depending on the circumstances of each.
Method 1: Relative Atomic Mass
The first method is to use the relative atomic mass scale. This is the most common method used by chemists. The relative atomic mass of an element is determined by comparing it to a standard atom. For example, the relative atomic mass of hydrogen is 1.008. This means that 1 gram of hydrogen has 1.008 times the mass of 1 atom of hydrogen.
Method 2: Atomic Mass Unit (amu)
The second method is to use the atomic mass unit, or amu. This is a unit of measure used to express the mass of atoms. The amu is equal to 1/12 the mass of a single atom of carbon-12. This means that the atomic mass of an element can be determined by multiplying the number of atoms by the amu of the element.
Method 3: Atomic Mass Formula
The third method is to use the atomic mass formula. This formula is used to calculate the atomic mass of an element by adding together the masses of its protons, neutrons, and electrons. The formula is:
Atomic mass = Mass of protons + Mass of neutrons + Mass of electrons
The atomic mass trick is a useful tool for chemists to accurately calculate the atomic masses of different elements. By using one of the three methods described above, chemists can easily calculate the atomic mass of any element.
What is atomic mass and how is it calculated?
Atomic mass is an important concept in chemistry and physics, as it helps us to understand the properties of different elements. In this article, we’ll look at what atomic mass is, why it’s important, and how it’s calculated.
What Is Atomic Mass?
Atomic mass is the sum of the masses of the protons, neutrons, and electrons in an atom, or the average mass, in a group of atoms. It is typically measured in unified atomic mass units (u), which is equal to 1/12 of the mass of a single carbon-12 atom.
Why Is Atomic Mass Important?
Atomic mass is important because it helps us to understand the properties of different elements. It tells us the mass of an individual atom or a group of atoms, which can help us to identify them. It also helps us to understand how different elements interact with each other, as elements with the same atomic mass are chemically similar.
How Is Atomic Mass Calculated?
There are several different methods for calculating atomic mass. The method you use depends on the information you’re given. Here are some of the most common methods:
By Referring to the Periodic Table
The periodic table is a chart of all the known elements, and it can be used to calculate atomic mass. Each element is represented by its symbol and its atomic number, which is the number of protons in the nucleus of an atom of that element. The atomic number is typically followed by a number in parentheses, which is the atomic mass of the element. For example, oxygen has an atomic number of 8 and an atomic mass of 16 (8).
By Adding the Masses of Protons and Neutrons
Another way to calculate atomic mass is by adding the masses of the protons and neutrons in the nucleus of the atom. The protons and neutrons have different masses, so you’ll need to use a periodic table to find the mass of each. Once you’ve added up the masses of the protons and neutrons, you’ll have the atomic mass of the atom.
By Accounting for the Mass Defect
The mass defect is the difference between the mass of an atom’s nucleus and the sum of the masses of its protons and neutrons. This difference is due to the energy released when the nucleons come together to form the nucleus. This energy is known as binding energy, and it can be used to calculate the atomic mass of an atom.
Atomic mass is an important concept in chemistry and physics, as it helps us to understand the properties of different elements. There are several different methods for calculating atomic mass, depending on the information you’re given. By understanding what atomic mass is and how it’s calculated, you’ll be better able to understand the properties of different elements.
How to calculate atomic mass?
Atomic mass is an important concept in both chemistry and physics. It is the sum of the masses of the protons, neutrons, and electrons in an atom or the average mass in a group of atoms. Knowing how to calculate atomic mass can help you understand the properties of different elements.
What Is Atomic Mass?
Atomic mass is the total mass of the protons, neutrons, and electrons in an atom. This is typically measured in atomic mass units (amu) or Dalton (Da). On the periodic table, an element’s atomic mass is the average mass of all of its isotopes. Isotopes are atoms of the same element with different numbers of neutrons.
Adding Protons and Neutrons
One way to calculate the mass of a single atom of an element is to consider the number of protons and neutrons present in its nucleus. The atomic mass of an atom is the sum of the masses of each proton and neutron present in its nucleus. This is also sometimes referred to as the mass number.
Calculating Atomic Mass
There are three main methods for calculating the atomic mass of an atom.
Using the Periodic Table
The periodic table is an excellent resource for finding the atomic mass of an element. Each element is represented by its atomic number, which is located just below the element’s symbol. The atomic mass is typically very similar to the element’s mass number, although there may be slight variations in the decimal places.
Using Atomic Mass Calculators
Atomic mass calculators can be used to quickly and easily calculate the atomic mass of an element. These calculators allow you to enter the element’s atomic number, number of protons and neutrons, and then calculate the atomic mass.
Using Atomic Mass Tables
Atomic mass tables are a great resource for finding the atomic mass of an element. These tables list the atomic mass of each element, as well as the atomic number, number of protons and neutrons, and other important information.
Understanding Atomic Mass
Understanding atomic mass can help you understand the properties of different elements. Knowing how to calculate atomic mass can also help you solve chemistry and physics problems. With the right resources, calculating atomic mass can be a relatively easy process.




Leave a Comment