Dynein Animation: Visualizing the Molecular Motor in Motion
Dynein animation vividly depicts the molecular motor’s directional stepping along microtubules by illustrating its structural dynamics and nucleotide-driven conformational changes. The animation conveys dynein’s power stroke, allosteric regulation, and cargo transport functions grounded in recent structural and biochemical evidence.
Understanding Dynein and Its Motility
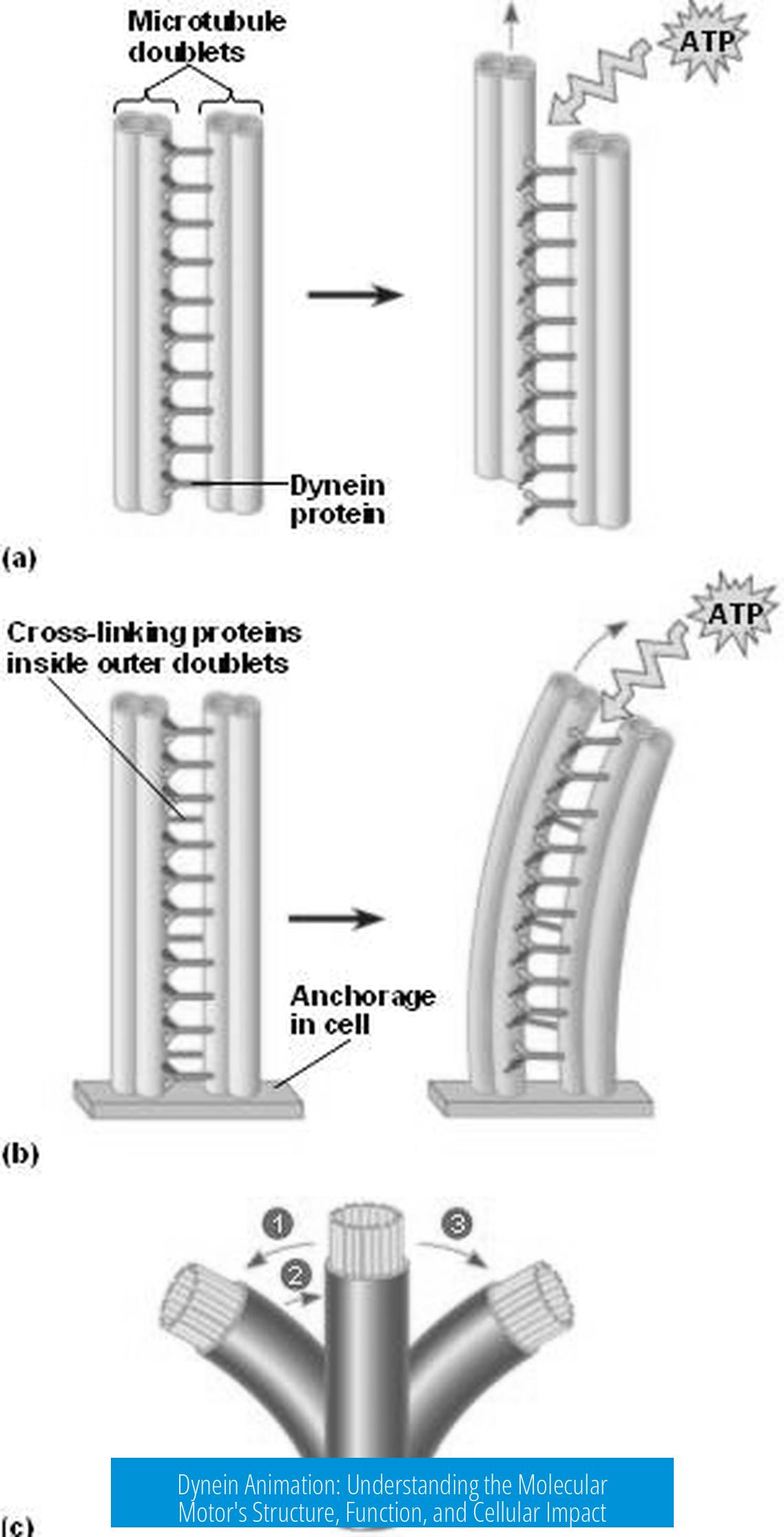
Dynein is a large motor protein complex that moves along microtubules inside cells. It ferries organelles and cargoes toward the microtubule’s minus end. Two forms of dynein exist:
- Axonemal dyneins, which drive cilia and flagella beating by sliding microtubules
- Cytoplasmic dynein, responsible for intracellular cargo transport and mitotic spindle functions
Animations focus primarily on cytoplasmic dynein’s stepping mechanism, helping visualize dynamic structural changes that are otherwise difficult to grasp from static images.
Dynein’s Structural Architecture for Animation
Motor Domain Components
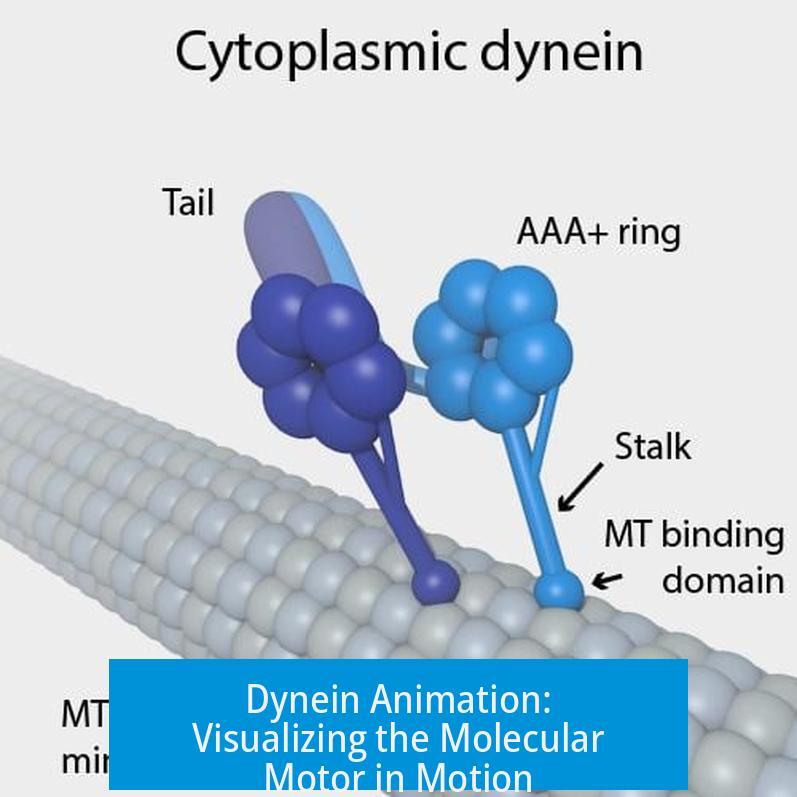
Each dynein heavy chain has two identical motor domains. Each motor domain contains:
- A hexameric AAA1–6 ATPase ring acting as the engine
- A stalk, a ~140 Å coiled coil projecting from the ring with a microtubule binding domain (MTBD) at the tip
- A buttress (or strut) that reinforces the stalk
- A helical linker domain that arches over the AAA ring, acting as a mechanical lever
The stalk transmits conformational changes between the AAA ring and the MTBD, controlling binding affinity to microtubules. The linker performs a critical swing motion that drives dynein’s power stroke.
Key Linker Conformations
Animation highlights the linker’s switching between bent and straight forms:
- Bent conformation: Observed in the ADP-vanadate trapped state, contacts the AAA2/3 interface, regarded as the pre-power stroke state
- Straight conformation: Found in the ADP state, the linker extends across the ring towards AAA4/5 and represents the post-power stroke state
This bent-to-straight linker swing is the force-generating stroke animators must emphasize to capture dynein’s mechanical step.
Nucleotide-Driven Conformational Changes in Dynein Animation
ATP binding and hydrolysis at the AAA1 site provoke large structural rearrangements essential for animation of dynein’s stepping cycle:
- ATP binding closes the AAA ring, causing linker bending (pre-power stroke)
- The buttress pulls on the stalk’s coiled coil, shifting it to a β+ registry and weakening MTBD’s affinity, allowing dynein to detach and reposition along the microtubule
- ATP hydrolysis releases phosphate, the AAA ring opens, stalk returns to α-registry, MTBD binds strongly, and linker straightens, producing the power stroke that moves the cargo forward
The animation translates these molecular transitions into a clear visual progression of steps, mirroring the motor’s biochemical rhythm.
Allosteric Regulation and Timing
Dynein’s AAA3 domain modulates the timing of conformational changes, critical for coordinated stepping:
- When ADP occupies AAA3, the AAA ring and linker undergo pronounced conformational shifts, allowing normal stepping
- When ATP (or analog AMPPNP) binds to AAA3, alterations are limited, the linker remains straight, and MTBD tightly binds microtubules, essentially locking dynein in a strong binding state
Including this allosteric control in animation clarifies how dynein coordinates ATP hydrolysis cycles with microtubule attachment and detachment, illustrating regulatory checkpoints that synchronize movement.
Mechanical Steps Illustrated in Animation
Animations break down dynein’s movement into distinct mechanical steps:
| Step | Conformational State | Effect on Microtubule Interaction | Key Animation Focus |
|---|---|---|---|
| ATP Binding | Closed AAA ring, linker bent | MTBD binds weakly (β+ registry), ready for detachment | Visualize linker bending and stalk registry shift |
| Detachment and Movement | Linker bent, stalk in weak state | Dynein releases the microtubule temporarily | Show dynein stepping along microtubule track |
| ATP Hydrolysis / Pi Release | AAA ring opens, linker straightens | MTBD binds strongly (α registry), power stroke occurs | Depict linker straightening and cargo movement |
| Preparation for Next Cycle | Reset to initial state | Dynein ready to bind ATP again and repeat | Loop animation cycle smoothly |
This sequence captures both the biochemical and mechanical essence of dynein stepping.
Contextual Comparison with Other Molecular Motors
Dynein is mechanistically unique compared to kinesin-1 and myosin V. While kinesins and myosins rely on small lever arms and neck linkers for movement, dynein utilizes the AAA ring and a large, swinging linker domain. Its complexity demands intricate animations to illustrate the allosteric communication between ATPase sites and microtubule interaction domains.
Animations can demonstrate dynein’s contrasting molecular design and functional role alongside kinesin by juxtaposing their stepping mechanics on microtubules.
Dynein and Kinesin in Cellular Transport Animation
In cell biology animations illustrating mitotic spindle assembly or intracellular trafficking, kinesins appear as orange motors transporting cargo along microtubules. Dyneins move with long, molecular “legs” transporting signals from the kinetochore. Highlighting the dynamics of dynein movement enriches understanding of mitotic checkpoint signaling and intracellular organization.
Summary of Dynein Animation Principles
- Dynein consists of homodimer motor domains with an AAA ring, stalk, buttress, and swinging linker.
- ATP binding closes the AAA ring and bends the linker, inducing weak microtubule binding for stepping.
- ATP hydrolysis opens the ring and straightens the linker, producing a power stroke and strong microtubule binding.
- AAA3 nucleotide state regulates allosteric control and timing of conformational changes.
- Animations visualize these motions as a cyclical stepping mechanism along microtubules.
- Dynein’s mechanism differs fundamentally from kinesin and myosin motors.
Key Takeaways
- Dynein animation traces conformational shifts of its AAA ring, stalk, and linker domains during ATP hydrolysis cycles.
- Animations use structural transitions such as linker’s bent-to-straight swing to depict power strokes driving motility.
- Allosteric regulation by AAA3 domain governs coordinated binding and stepping frequency.
- Dynein’s complex motor domain architecture contrasts with other molecular motors, influencing animation style and focus.
- Animations clarify dynein’s role in cargo transport and mitotic spindle function by showing directional microtubule stepping.
Dynein Animation: Unfolding the Molecular Dance of Cellular Motors
What exactly is dynein animation, and why is it a big deal in understanding cellular motion? Simply put, dynein animation visually demonstrates how the dynein motor protein powers its way along microtubule tracks inside cells. These animations aren’t just flashy graphics. They reveal the detailed mechanics of how dynein “walks” — a process crucial for transporting vital cargos within cells.
Dynein motors are like the cellular freight trucks, delivering organelles, proteins, and even viruses to precise destinations. Understanding their intricate movements through animation gives us a window into the molecular machinery that maintains cellular traffic and health.
Dynein in a Nutshell: The Cellular Motor You Didn’t Know You Knew
Dynein isn’t just one motor — it’s a family divided mainly into two types: axonemal and cytoplasmic. Axonemal dyneins work in cilia and flagella, the tiny hair-like structures cells use for movement or fluid flow. Cytoplasmic dynein, our star player in animation, carries cargos along microtubules, mostly heading toward the microtubule’s minus end (think “cell center”).
Animation models highlight dynein’s directional bias; it steps purposefully, resisting the temptation to wander aimlessly through the crowded cellular cytoplasm.
Structural Secrets Worth Animating
Diving under the hood, dynein’s motor domain is a homodimer — two identical halves working in tandem. Each holds a complex hexameric AAA ring from which three key structures protrude: the stalk, the buttress, and the linker. These aren’t just fancy names, they’re the parts responsible for dynein’s movements.
- The stalk is a long coiled coil about 140 angstroms in length, tipped with the microtubule-binding domain (MTBD), which clutches the track.
- The buttress (also called the strut) supports the stalk, helping it shift between “strong” and “weak” binding states.
- The linker arches over the AAA ring like a mechanical lever, swinging between bent and straight positions — the main animation highlight.
In animation terms, these structural nuances allow creators to depict dynein’s power stroke, a pivotal event driving its step.
The Nucleotide Cycle: Dynein’s Fuel and Motion Control
ATP binding and hydrolysis power dynein’s movement. When ATP binds at the AAA1 site on the ring, it triggers the ring to close like a flower bud. This closure causes the linker to bend into a “pre-power stroke” state. The buttress and stalk respond by shifting coarse coils, weakening the MTBD’s grasp on the microtubule track and setting the stage for movement.
After ATP hydrolysis, dynein “releases” the energy, straightening the linker into the “post-power stroke” configuration. This shift pushes the stalk back into a strong binding mode and reopens the AAA ring, anchoring the motor firmly to the microtubule and completing the step. Animators capture this process as an elegant “swinging lever” motion — a visual feast of molecular engineering.
Timing is Everything: The Regulatory Role of AAA3
The AAA3 domain plays the role of molecular traffic controller in this animation saga. Its nucleotide state influences timing, coordinating how strongly dynein clings to microtubules and when it moves.
- When ADP is bound at AAA3, large conformational changes ripple through the entire AAA ring and linker, priming dynein to proceed.
- When ATP (or analog AMPPNP) binds AAA3, the ring’s changes are limited, the linker stays straight, and the MTBD clamps tightly to the microtubule.
This layered regulation is perfect animation material, highlighting that dynein’s steps are not just simple pushes but intricately timed with nucleotide signals.
Mechanical Steps: Talking Power Strokes and Stepping Dynamics
Animating dynein’s journey is about showing dynamic transitions, not frozen snapshots.
- Start with the bent linker in the ATP-bound, pre-power stroke state, where dynein loosens its grip.
- Then depict the stalk sliding to weaken microtubule binding, enabling dynein to take a step.
- Finally, show ATP hydrolysis driving the linker back to a straight posture with strong microtubule attachment — the power stroke finishes the step.
This cycle repeats, producing processive movement. Unlike kinesin’s “walking” gait, dynein utilizes these conformational changes as its unique “gear-shifts” to walk the cellular highways.
How Dynein Stacks Up Against Other Molecular Motors
For perspective, kinesin-1 and myosin V are the stars of cargo transport, well-charted through decades of study. They’re straightforward walking motors with rigid stepping sequences. Dynein is the complex cousin at the family reunion — it uses a ring-shaped ATPase domain and a swinging linker rather than a simple lever-like neck domain.
Animations contrasting these motors amplify the unique structural and mechanistic traits of dynein, pulling viewers deeper into the molecular ballet that powers life inside cells.
Bringing Dynein to Life: The Power of Animation in Research and Education
Why bother animating something so tiny?
Scientific publications and data are often bogged down by dense jargon and still images. Animation transforms these static models into dynamic, intuitive stories. It helps researchers spot and refine hypotheses about dynein’s mechanics and coordination with partners like dynactin, a large multi-subunit complex that beefs up dynein’s motility.
For educators and students, dynein animations turn abstract molecular biology into memorable scenes — revealing how dynein carries mitotic checkpoint proteins from kinetochores to spindle poles during cell division, or how its missteps might contribute to brain developmental defects.
Telling the Story Through Animation: Tips for Accurate Visualizations
Good dynein animations pay attention to allosteric timing, stalk registry shifts, and the nuanced choreography of the linker domain. Animators balance accuracy with clarity, often simplifying the model while maintaining key transitions:
- Show the AAA ring breathing: closed in ATP-bound state, open when ADP binds.
- Animate the stalk’s registry shift: α to β+ to α, signaling strong or weak microtubule binding.
- Highlight the linker’s swing: bent pre-power stroke, straight post-power stroke.
Combining these elements with current crystal and cryo-EM structures, plus single molecule kinetics, yields an informative, engaging animation that resonates with viewers without sacrificing scientific rigor.
Real-Life Applications: Why Dynein Animation Matters
Understanding dynein’s stepping mechanism helps in multiple fields.
- Biomedical research: Faulty dynein function links to neurodegenerative diseases and developmental brain disorders.
- Drug design: Targeting dynein’s motor and regulatory domains could lead to therapies that modulate intracellular transport.
- Nanotechnology: Inspiration from dynein’s mechanics can fuel the design of synthetic molecular machines.
Animations provide the blueprint for researchers and engineers to imagine interventions and innovations based on dynein’s precise, nucleotide-driven steps.
Putting It Together: Dynein’s Animation as a Molecular Performance
Picture this: The cytoplasmic dynein homodimer, each motor domain with its AAA ring, stalk, buttress, and linker, takes turns unleashing power strokes. ATP binds, the ring clamps down, the linker bends, stalk loosening its grip — a molecular pinch and release. ATP hydrolysis flips the switch, linker snaps straight, the stalk grabs tight again, and the motor shuffles forward.
This isn’t just abstract science — it’s a molecular performance, choreographed by nucleotides and allosteric signals, rendered vividly in animations that make the tiny giants of cellular transport come alive.
Ready to Dive Deeper?
For anyone fascinated by cell biology, molecular motors, or animation technology, digging into dynein animations opens up a vibrant world. The interplay of structure, chemistry, and mechanics is as intricate as any cinematic dance. And with ongoing advances in cryo-EM and single molecule imaging, these animations will only get richer and more insightful.
So next time you see an animation of dynein striding along a microtubule, appreciate the molecular precision packed inside that elegant swing — a marvel of nature’s nanotechnology at work inside every living cell.
What structural features of dynein are key for animation depiction?
Dynein’s motor domain includes a hexameric AAA ring with three key parts: stalk, buttress, and linker. The linker changes shape during movement, swinging from bent to straight, which acts as a power stroke. These shifts are critical for animating its stepping motion.
How do nucleotide states control dynein’s movements in animation?
ATP binding causes the AAA ring to close, shifting the stalk to a weak binding state. After ATP hydrolysis, the stalk returns to strong binding and the linker straightens. Animations use these nucleotide-triggered changes to show dynein’s stepping along microtubules.
Why is the linker conformational change important in dynein animation?
The linker swings from bent in the pre-power stroke state to straight in the post-power stroke state. This mechanical shift is the core power stroke driving dynein’s movement and is visually emphasized when animating dynein’s motion.
How is dynein movement different from other motor proteins in animations?
Unlike kinesin or myosin that use simpler mechanisms, dynein’s motion relies on complex AAA ring conformational changes and linker swings. Animations highlight these unique structural shifts to show how dynein moves directionally along microtubules.
What roles do the stalk and buttress play in dynein animations?
The stalk binds to microtubules, switching between strong and weak binding states controlled by buttress-induced shifts. Animations illustrate how the buttress pulling on the stalk’s coiled coil enables dynein to grip and release the microtubule during stepping.
How does allosteric regulation affect dynein animations?
Nucleotide binding at AAA3 modulates the motor’s conformational changes. ATP at AAA3 keeps the linker straight and microtubule binding strong, while ADP allows larger ring and linker shifts. Animations reflect this regulation by showing timing and strength of dynein’s microtubule interactions.




Leave a Comment