Atoms are the building blocks of the universe; they make up everything from the air we breathe to the stars in the sky. Every atom has protons and neutrons at its core, the positively charged protons and the neutral neutrons. Together, these two subatomic particles give an atom its mass, and when combined with electrons, an atom’s chemical properties are determined. But what happens when an atom has a different number of protons and neutrons?
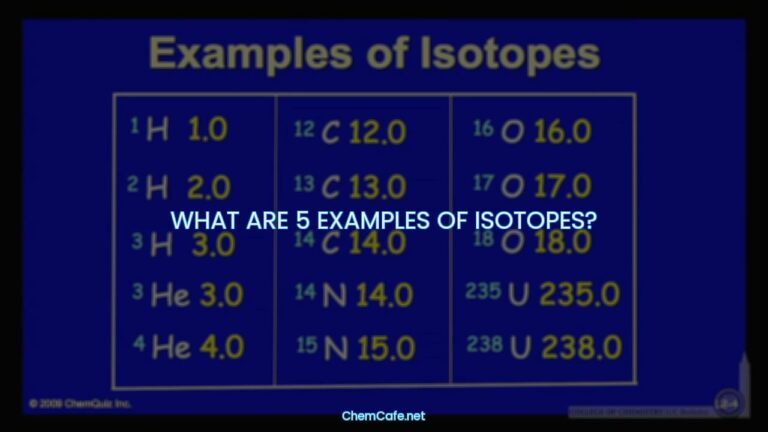
Isotopes are atoms of the same element with different numbers of neutrons in their nuclei. These isotopes are identified by their mass, which is the total number of protons and neutrons. By changing the number of neutrons in an atom, it does not change the element, but it does change the mass of the element.
But what if we wanted to know what element has 12 neutrons and 11 protons? Well, the answer is actually quite simple. Atoms with 12 neutrons and 11 protons are examples of the element carbon. Carbon is one of the most common elements on Earth and is found in all living things. It is one of the most abundant elements in the universe and makes up around 18% of the Earth’s crust.
Carbon is a very versatile element, with three different isotopes. Each isotope has 6 protons, since they are all carbon atoms, but the number of neutrons varies across the three isotopes. The atom on the left has 6 neutrons, the atom in the middle has 7 neutrons, and the atom on the right has 8 neutrons.
So, when looking for the element with 12 neutrons and 11 protons, the answer is carbon. With its versatile nature, carbon is a key component of many things, from the air we breathe to the stars in the sky. Its ability to form different isotopes is what makes it so unique and useful. So the next time you want to know what element has 12 neutrons and 11 protons, you now know the answer is carbon.
What element has 12 neutrons and 11 protons?
Isotopes are atoms of the same element with different numbers of neutrons in their nuclei. They have the same number of protons and electrons, but a different number of neutrons. Changing the number of neutrons in an atom does not change the element, but does change the mass of the element. Isotopes can be identified by their mass, which is the total number of protons and neutrons.
Naming Isotopes
Isotopes are generally written using two methods; both use the mass of the atom, where mass = (number of protons) + (number of neutrons). For example, an atom with 6 protons and 6 neutrons will be written as 12C, and an atom with 6 protons and 7 neutrons will be written as 13C. The number before the C stands for the mass of the atom, and the C stands for the element, carbon.
What Element Has 12 Neutrons and 11 Protons?
The element with 12 neutrons and 11 protons is sodium-23, or 23Na. Sodium-23 has 11 protons and 12 neutrons, and a mass of 23 (11 + 12). Sodium-23 is an isotope of sodium, and it is the most abundant isotope of sodium.
What is the Difference Between Sodium-23 and Sodium-24?
The difference between sodium-23 and sodium-24 is the number of neutrons. Sodium-23 has 12 neutrons and sodium-24 has 13 neutrons. Both isotopes have 11 protons, but the additional neutron in sodium-24 gives it a mass of 24.
What is the Difference Between Sodium-23 and Sodium-25?
The difference between sodium-23 and sodium-25 is the number of neutrons. Sodium-23 has 12 neutrons and sodium-25 has 14 neutrons. Both isotopes have 11 protons, but the additional two neutrons in sodium-25 give it a mass of 25.
Uses of Sodium-23
Sodium-23 is used in a variety of applications. It is used in the production of medical isotopes for diagnostic and therapeutic purposes, as well as in nuclear power plants as a source of energy. In addition, it is used in research to study the effects of radiation on living organisms.
The element with 12 neutrons and 11 protons is sodium-23, or 23Na. Sodium-23 has 11 protons and 12 neutrons, and a mass of 23. Sodium-23 is an isotope of sodium and is used in a variety of applications, including medical isotopes, nuclear power plants, and research studies.
What atom has 11 protons and 12 neutrons?
Atoms are the smallest particles of matter that make up everything in the universe. They are made up of protons, neutrons, and electrons. The number of protons and neutrons in each atom determines the element it belongs to, and the number of electrons determines the atom’s charge.
Atomic Structure
Atoms consist of a nucleus, which contains protons and neutrons, and electrons that orbit around the nucleus. The number of protons in an atom’s nucleus determines its element, so all atoms of a given element have the same number of protons. The number of neutrons can vary, however, and these variations are known as isotopes.
Isotopes are atoms of the same element that have different numbers of neutrons in their nuclei. This means that atoms of the same element can have different masses. Atoms are electrically neutral, so they have the same number of negatively-charged electrons as positively-charged protons.
Identifying Isotopes
Isotopes are identified by their mass, which is the total number of protons and neutrons. There are two ways that isotopes are generally written. They both use the mass of the atom where mass = (number of protons) + (number of neutrons).
Sodium
Let’s look at a sodium atom as an example. Sodium has 11 protons and 12 neutrons in its nucleus, and 11 electrons outside the nucleus. Sodium is one of several atoms that easily donates an electron. This leaves just 10 electrons, but since there are still 11 protons, sodium has a plus one charge. This charge means it’s a sodium ion. Certain other elements tend to gain electrons.
Carbon Isotopes
Here we see three isotopes of carbon. They all have 6 protons, since they’re all carbon atoms. The atom on the left has 6 neutrons, the atom in the middle has 7 neutrons, and the atom on the right has 8 neutrons. This means that the masses of these three atoms are 12, 13, and 14, respectively.
Conclusion
To answer the question, the atom that has 11 protons and 12 neutrons is sodium. It is an isotope of sodium, and its mass is 23. Sodium is a very important element in the universe, and it has a wide range of uses. It is found naturally in many foods, and it is a necessary electrolyte for the human body.
Atoms and their isotopes play a crucial role in the universe. Knowing the number of protons and neutrons in an atom can help us identify the element and its isotopes. This knowledge is essential for understanding the properties of matter and how it interacts with other elements.
Which element has 12 neutrons and 11 electrons?
Atoms of different elements can have different numbers of protons, neutrons, and electrons. This is why certain elements have different isotopes. Isotopes are atoms with the same number of protons, but different numbers of neutrons. For example, the element sodium has 11 protons and 12 neutrons in its nucleus, and 11 electrons outside the nucleus. This means that sodium is one of several elements that easily donate an electron, leaving just 10 electrons, but since there are still 11 protons, sodium has a plus one charge.
In other words, sodium is an ion, and its isotopes are atoms that have the same number of protons and electrons, but a different number of neutrons. To illustrate, let’s take a look at three isotopes of carbon. All three have 6 protons, but the atom on the left has 6 neutrons, the atom in the middle has 7 neutrons, and the atom on the right has 8 neutrons.
Changing the number of neutrons in an atom does not change the element, but it does change the mass of the element. This is why certain elements have different isotopes. For instance, sodium with 11 protons and 12 neutrons has a mass of 23, while sodium with 11 protons and 14 neutrons has a mass of 25.
Naming Isotopes
Atoms can have different numbers of neutrons and thus have different masses, but they still remain the same element. This is why it is important to know how to name isotopes. There are two common ways to name isotopes. The first is to use the element’s name, followed by the mass number of the isotope. For example, sodium-23, sodium-25, and sodium-27 are all isotopes of sodium.
The second way to name isotopes is to use the element’s name, followed by the number of protons and neutrons in the nucleus. For example, sodium-11-12 and sodium-11-14 are both isotopes of sodium. In this case, sodium-11-12 has 11 protons and 12 neutrons, while sodium-11-14 has 11 protons and 14 neutrons.
In conclusion, isotopes are atoms of the same element with different numbers of neutrons in their nuclei. Isotopes can be identified by their mass numbers or the number of protons and neutrons in their nuclei. For instance, sodium-11-12 has 11 protons and 12 neutrons, while sodium-11-14 has 11 protons and 14 neutrons. Therefore, the element with 12 neutrons and 11 electrons is sodium.
What element has 11 protons 9 neutrons and 11 electrons?
Atoms are the basic building blocks of matter and the structure of atoms is determined by the number of protons, neutrons, and electrons they contain. Each element has a unique atomic structure, and understanding this structure is key to understanding the properties of that element. Sodium is the element that has 11 protons, 9 neutrons, and 11 electrons.
The Number of Protons
The number of protons in an atom determines the atomic number of the element. The atomic number of an element is the number of protons it has. Sodium has an atomic number of 11, meaning it has 11 protons in its nucleus. The 11 protons have a positive charge of +11, while the 10 electrons have a negative charge of -10. This results in a net charge of +1.
The Mass Number
The mass number of an element tells us the number of protons AND neutrons in an atom (the two particles that have a measurable mass). The most common isotope of sodium has a mass number of 23amu, which tells us that sodium has 11 protons and 12 neutrons. This means that sodium has 11 protons, 12 neutrons, and 11 electrons.
Isotopes
Isotopes are atoms of the same element with different numbers of neutrons in their nuclei. For example, carbon has three different isotopes, each with 6 protons, since they are all carbon atoms. Atoms are electrically neutral, so they have the same number of negatively charged electrons as positively charged protons. The atom on the left has 6 neutrons, the atom in the middle has 7 neutrons, and the atom on the right has 8 neutrons.
In conclusion, sodium is the element that has 11 protons, 9 neutrons, and 11 electrons. This atom has an atomic number of 11 and a mass number of 23amu. Isotopes of an element have the same number of protons but different numbers of neutrons, which affects the mass of the atom. Understanding the atomic structure of an element is essential to understanding its properties.
What has 12 neutrons and 11 electrons?
We all know that atoms are made up of protons, neutrons, and electrons. But how do you know how many of each an atom contains? The answer lies in its atomic number and mass number. The atomic number of an atom tells us the number of protons present in its nucleus, while the mass number tells us the total number of protons and neutrons.
The element Sodium (Na) has an atomic number of 11 and a mass number of 23. This means that Sodium has 11 protons and 12 neutrons in its nucleus, and 11 electrons outside the nucleus. The number of protons and electrons are the same, so they cancel each other out, resulting in a neutral atom.
What is an Isotope?
An isotope is an atom of an element with the same number of protons as other atoms of that element, but different numbers of neutrons. This means that the atomic number remains the same, but the mass number changes. Isotopes can have different physical and chemical properties, depending on the number of neutrons they contain.
What are the Subatomic Particles in the Nucleus?
The nucleus of an atom is made up of two subatomic particles: protons and neutrons. Protons are positively charged particles, while neutrons are neutral particles with no charge. Together, they form the nucleus of an atom.
What Does the Atomic Number Represent?
The atomic number (the smaller number) tells us the number of protons in the nucleus of that atom. This number is always the same for all atoms of a particular element. For example, all atoms of the element Sodium have 11 protons in their nucleus.
In conclusion, the element Sodium has an atomic number of 11 and a mass number of 23. This means that Sodium has 11 protons, 12 neutrons, and 11 electrons. The number of protons and electrons are the same, resulting in a neutral atom. Isotopes are atoms of the same element that have different numbers of neutrons, which can affect their physical and chemical properties. Finally, the atomic number represents the number of protons in the nucleus of an atom.
What element has 11 protons?
Atoms are the building blocks of all matter, and each element has its own unique atom. The number of protons in the nucleus of an atom is the atomic number of the element and determines what element it is.
One element with 11 protons is sodium, which has the chemical symbol Na. Sodium is a very reactive element, and is often found in compounds rather than in its pure form. It is an essential element for life and is found in many foods and is a major component of table salt.
What is an Isotope?
An isotope is an atom of the same element with a different number of neutrons in its nucleus. All atoms of a given element will have the same number of protons, but the number of neutrons can vary. This gives each isotope of an element a different mass number, which is the total number of protons and neutrons in the nucleus.
For example, the isotopes of carbon are carbon-12, carbon-13, and carbon-14. All three isotopes have six protons, but carbon-12 has six neutrons, carbon-13 has seven neutrons, and carbon-14 has eight neutrons. Because they all have the same number of protons, they are all carbon atoms.
What is the Number of Protons and Neutrons in Sodium?
The element sodium has 11 protons and 12 neutrons in its nucleus. This gives it a mass number of 23, which is the sum of its protons and neutrons. Because all atoms of an element have the same number of protons, all atoms of sodium have 11 protons. The number of electrons outside the nucleus is also 11, since atoms are electrically neutral and have the same number of positively charged protons and negatively charged electrons.
Atoms of an element all have the same number of protons, which is the atomic number of the element. This means that an element with 11 protons is sodium, which has a mass number of 23, 11 protons, and 12 neutrons. Isotopes are atoms of the same element with different numbers of neutrons, which gives them different mass numbers. Understanding isotopes and how they differ from each other is an important part of chemistry.



Leave a Comment