The electron configuration of an element is a notation system used to describe how electrons are distributed among the various atomic orbitals. This notation is essential in modern chemistry, since it helps us to predict the properties of a group of elements, understand their valency, and interpret atomic spectra.
The electron configuration of an element is based on the periodic table, which arranges elements in order of increasing atomic number (Z). This table also shows the electron configuration for each subshell. Starting from the left, with hydrogen (Z=1), the filling order simply progresses in increasing atomic number (Z).
The standard notation for electron configurations often yields lengthy results, especially for elements with larger atomic numbers. This can be attributed to the fact that electrons are distributed among multiple orbitals, each with a specific number of electrons. For example, the electron configuration of sodium is 1s22s22p63s1.
The electron configuration of an element is an essential part of modern chemistry, and it plays a vital role in determining the properties of a group of elements. It is also used to predict the valency of an element and interpret atomic spectra. Knowing the electron configuration of an element can help us better understand its structure and properties, as well as its role in the periodic table.
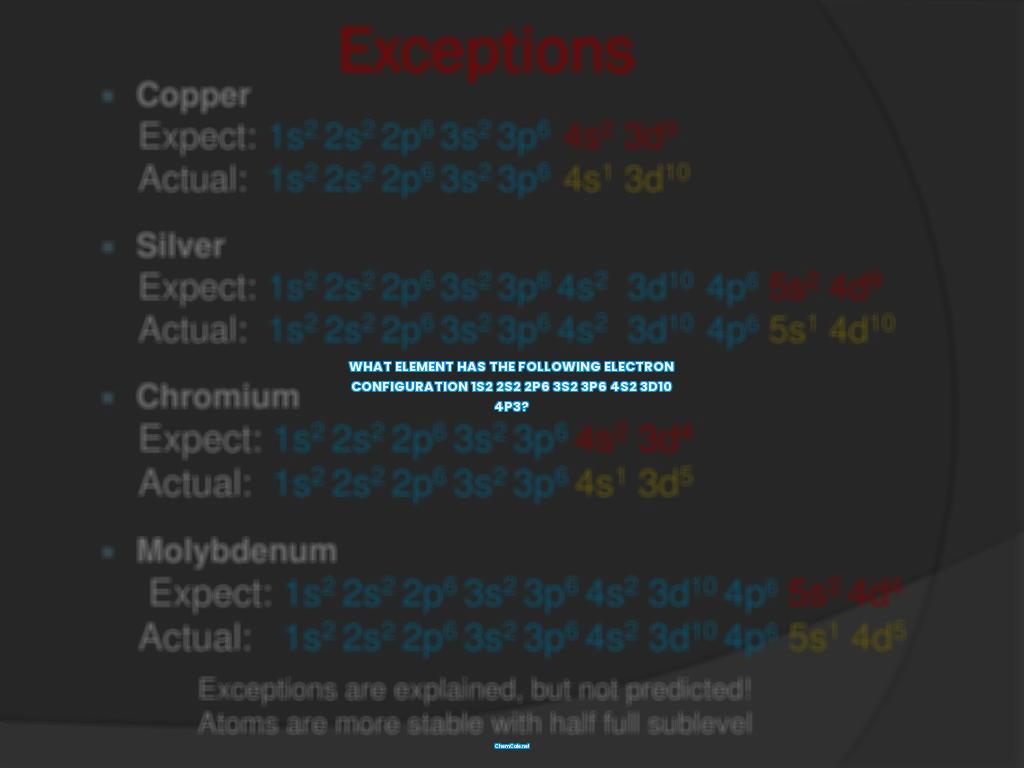
What element has the following electron configuration 1s2 2s2 2p6 3s2 3p6 4s2 3d10 4p3?
Atoms have a unique arrangement of electrons in their orbitals, which is known as their electron configuration. Electron configurations are useful for determining the valency of an element, predicting the properties of a group of elements, and interpreting atomic spectra. The electron configuration of an element is described using a standard notation, in which all electron-containing atomic subshells (with the number of electrons they hold written in superscript) are placed in a sequence.
For example, the electron configuration of sodium is 1s22s22p63s1. When examining the electron configuration of an element, the first number indicates the energy level, followed by the type of orbital, and then the number of electrons in the orbital.
So, the electron configuration 1s2 2s2 2p6 3s2 3p6 4s2 3d10 4p3 is the configuration for the element Chromium (Cr). The first number (1) indicates that the electrons are in the first energy level, the s indicates that they are in the s orbital, and the 2 indicates that there are two electrons in the s orbital.
The second number (2) indicates that the electrons are in the second energy level, the s indicates that they are in the s orbital, and the 2 indicates that there are two electrons in the s orbital. The third number (2) indicates that the electrons are in the third energy level, the p indicates that they are in the p orbital, and the 6 indicates that there are six electrons in the p orbital.
The fourth number (2) indicates that the electrons are in the fourth energy level, the s indicates that they are in the s orbital, and the 2 indicates that there are two electrons in the s orbital. The fifth number (3) indicates that the electrons are in the fifth energy level, the d indicates that they are in the d orbital, and the 10 indicates that there are ten electrons in the d orbital.
Finally, the sixth number (3) indicates that the electrons are in the sixth energy level, the p indicates that they are in the p orbital, and the 3 indicates that there are three electrons in the p orbital. Thus, the electron configuration 1s2 2s2 2p6 3s2 3p6 4s2 3d10 4p3 is the electron configuration for the element Chromium (Cr).
In addition to determining the electron configuration of an element, electron configurations can also be used to predict the properties of a group of elements. Elements with similar electron configurations tend to exhibit similar properties, so knowing the electron configuration can help predict what properties an element will have.
Finally, electron configurations can also be used to interpret atomic spectra. Atomic spectra are the light emitted from an atom when it is excited. By studying the wavelength of the light emitted, the electron configuration of an atom can be determined.
In summary, the electron configuration 1s2 2s2 2p6 3s2 3p6 4s2 3d10 4p3 is the electron configuration for the element Chromium (Cr). Electron configurations are useful for determining the valency of an element, predicting the properties of a group of elements, and interpreting atomic spectra.
Which element has the following electron configuration 1s2 2s2 2p6 3s2 3p6 4s2 3d10 quizlet?
Electron configurations are an essential part of understanding the behavior of atoms and molecules. They are used to predict the properties of elements, determine the valency of an element, and interpret atomic spectra. This notation for the distribution of electrons in the atomic orbitals of atoms came into practice shortly after the Bohr model of the atom was presented by Ernest Rutherford and Niels Bohr in the year 1913.
What are Electron Configurations?
The electron configuration of an element describes how electrons are distributed in its atomic orbitals. Electron configurations of atoms follow a standard notation in which all electron-containing atomic subshells (with the number of electrons they hold written in superscript) are placed in a sequence. For example, the electron configuration of sodium is 1s22s22p63s1.
Table of Content
However, the standard notation often yields lengthy electron configurations (especially for elements having a relatively large atomic number). Since the arrangement of the periodic table is based on the electron configurations, Figure (PageIndex{3}) provides an alternative method for determining the electron configuration. The filling order simply begins at the top left, with hydrogen (Z=1) and includes each subshell as you proceed in increasing atomic number (Z) order.
Figure (PageIndex{3}:
This periodic table shows the electron configuration for each subshell. As you can see, the electron configuration 1s2 2s2 2p6 3s2 3p6 4s2 3d10 is associated with the element Copper (Cu), which has an atomic number of 29. Copper belongs to the group 11 elements (also known as the coinage metals) and has a valency of 2.
The electron configuration 1s2 2s2 2p6 3s2 3p6 4s2 3d10 is also associated with the element Silver (Ag), which has an atomic number of 47. Silver belongs to the group 11 elements (also known as the coinage metals) and has a valency of 1.
The electron configuration 1s2 2s2 2p6 3s2 3p6 4s2 3d10 is also associated with the element Gold (Au), which has an atomic number of 79. Gold belongs to the group 11 elements (also known as the coinage metals) and has a valency of 3.
In conclusion, the electron configuration 1s2 2s2 2p6 3s2 3p6 4s2 3d10 is associated with the elements Copper (Cu), Silver (Ag), and Gold (Au). All three of these elements belong to the group 11 elements (also known as the coinage metals) and have valencies of 2, 1, and 3, respectively. Understanding electron configurations is essential for predicting the properties of elements and interpreting atomic spectra.
Which element has the following electron configuration 1s2 2s2 2p6 3s2 3p6 4s1?
Atoms are made up of protons, neutrons, and electrons. Electron configurations provide a way to describe the arrangement of electrons in an atom. The electron configuration of an atom describes how electrons are distributed in its atomic orbitals, which helps determine the properties of the element.
The electron configuration of an element follows a standard notation in which all electron-containing atomic subshells (with the number of electrons they hold written in superscript) are placed in a sequence. For example, the electron configuration of sodium is 1s22s22p63s1.
The specific electron configuration in the question is 1s2 2s2 2p6 3s2 3p6 4s1. This electron configuration belongs to the element Silicon (Si). Silicon has an atomic number of 14, which means it has 14 protons and 14 electrons.
Understanding Electron Configurations
In order to understand electron configurations, it is important to understand the structure of an atom. Atoms are made up of a nucleus, which is composed of protons and neutrons, and a cloud of electrons surrounding the nucleus. The electrons are arranged in energy levels or shells and each shell is further divided into subshells or orbitals.
The electrons in each shell or subshell are arranged in a specific order, known as the electron configuration. The standard notation for electron configurations is a sequence of atomic subshells (with the number of electrons they hold written in superscript). This notation is used to describe the distribution of electrons in the atomic orbitals of atoms.
Silicon’s Electron Configuration
Silicon has an atomic number of 14, which means it has 14 protons and 14 electrons. The electron configuration of Silicon is 1s2 2s2 2p6 3s2 3p6 4s1. This means that the electrons are distributed in the following order:
The first energy level (shell) holds two electrons in its single subshell (1s2).
The second energy level (shell) holds eight electrons, with two in its first subshell (2s2) and six in its second subshell (2p6).
The third energy level (shell) holds eight electrons, with two in its first subshell (3s2) and six in its second subshell (3p6).
The fourth energy level (shell) holds two electrons in its single subshell (4s1).
Uses of Electron Configurations
Electron configurations are useful for a variety of purposes, including determining the valency of an element, predicting the properties of a group of elements, and interpreting atomic spectra. Electron configurations can also be used to explain the arrangement of elements in the periodic table.
The arrangement of the periodic table is based on electron configurations and the filling order simply begins at the top left, with hydrogen (Z=1) and includes each subshell as you proceed in increasing atomic number (Z) order.
In conclusion, the electron configuration 1s2 2s2 2p6 3s2 3p6 4s1 belongs to the element Silicon (Si). Electron configurations provide a way to describe the arrangement of electrons in an atom, which helps determine the properties of the element. Electron configurations are also useful for determining the valency of an element, predicting the properties of a group of elements, and interpreting atomic spectra.
Which element has an electron configuration of 1s2 2s2 2p6 3s2 3p6 4s2 3d10 4p3?
Electron configurations are a useful way to understand the properties of an element. They provide information about the number of electrons in an atom, how the electrons are distributed in the atom’s orbitals, and the valency of the element. One such electron configuration is 1s2 2s2 2p6 3s2 3p6 4s2 3d10 4p3, which belongs to a particular element.
What is an electron configuration?
An electron configuration is a notation used to describe the distribution of electrons in an atom’s orbitals. It consists of a sequence of atomic subshells, written in superscript, with the number of electrons they contain. This notation was developed shortly after the Bohr model of the atom was proposed in 1913.
What is the electron configuration of 1s2 2s2 2p6 3s2 3p6 4s2 3d10 4p3?
The electron configuration of 1s2 2s2 2p6 3s2 3p6 4s2 3d10 4p3 represents the distribution of electrons in the orbitals of an atom. This configuration can be broken down into several components. The 1s2 2s2 2p6 3s2 3p6 part of the configuration tells us that there are two electrons in the 1s orbital, two in the 2s orbital, and six in the 2p orbital. The 4s2 3d10 4p3 part of the configuration tells us that there are two electrons in the 4s orbital, ten in the 3d orbital, and three in the 4p orbital.
Which element has an electron configuration of 1s2 2s2 2p6 3s2 3p6 4s2 3d10 4p3?
The electron configuration of 1s2 2s2 2p6 3s2 3p6 4s2 3d10 4p3 belongs to the element copper (Cu). Copper is a transition metal located in the d-block of the periodic table. It has an atomic number of 29 and an atomic mass of 63.546 amu. Copper is a malleable and ductile metal with a reddish-orange color. It is an important component of many alloys and is used in electrical wiring and plumbing.
What are the properties of copper?
Copper has a number of unique properties that make it an important material in many industries. It is an excellent conductor of heat and electricity, and is highly corrosion-resistant. Copper is also a relatively soft metal, making it easy to shape. Additionally, it is antimicrobial, meaning it can kill bacteria and other microorganisms. Copper is also very malleable and ductile, meaning it can be easily manipulated into a variety of shapes and sizes.
What else can we learn from the electron configuration of copper?
The electron configuration of copper can also tell us about its valency. Valency is the number of electrons in the outermost shell of an atom. In the case of copper, the valency is 1, which means it can form one bond with another element. This is what makes copper useful in alloys and electrical wiring. Copper’s electron configuration can also tell us about its reactivity. Copper is a relatively unreactive element, meaning it does not easily react with other elements.
The electron configuration of 1s2 2s2 2p6 3s2 3p6 4s2 3d10 4p3 belongs to the element copper. This configuration tells us about the number of electrons in the atom, how they are distributed in the atom’s orbitals, and the valency of the element. It also tells us about copper’s reactivity and its usefulness in alloys and electrical wiring. Understanding electron configurations is important for predicting the properties of elements and interpreting atomic spectra.
Which of the following elements has the electron configuration 1s2 2s2 2p6 3s2 3p1?
When dealing with elements, it’s important to understand their electron configurations. Electron configurations describe the distribution of electrons in an atom’s atomic orbitals. This information can be used to identify elements, predict their properties, and understand their behavior.
The standard notation for an element’s electron configuration is a sequence of numbers and letters that indicate the subshells and the number of electrons contained in each. For example, the electron configuration of sodium is 1s22s22p63s1.
However, when dealing with elements of higher atomic numbers, this notation can become quite lengthy. To make things easier, the periodic table can be used to determine an element’s electron configuration. Starting at the top left with hydrogen (Z=1), each subshell is included as the atomic number increases.
Now, let’s answer the question: which of the following elements has the electron configuration 1s2 2s2 2p6 3s2 3p1? To answer this, we’ll look at the electron configurations of magnesium (Z=12) and silicon (Z=14).
The electron configuration of magnesium is 1s2 2s2 2p6 3s2. This matches the electron configuration given in the question, so the answer is magnesium.
The electron configuration of silicon, on the other hand, is 1s2 2s2 2p6 3s2 3p2. This does not match the electron configuration given in the question, so the answer is not silicon.
In conclusion, the element with the electron configuration 1s2 2s2 2p6 3s2 3p1 is magnesium. Knowing how to interpret electron configurations can be incredibly useful, as it can be used to identify elements, predict their properties, and understand their behavior.
Which of the following elements has the electron configuration 1s22s22p63s23p3?
Electron configurations are important in understanding the properties of atoms and molecules. They provide insights into the electronic structure of atoms, which is essential for understanding the behavior of molecules. Specifically, the electron configuration of an element describes how electrons are distributed in its atomic orbitals.
What is an Electron Configuration?
An electron configuration is a notation for describing the arrangement of electrons in an atom. It follows a standard notation in which all electron-containing atomic subshells (with the number of electrons they hold written in superscript) are placed in a sequence. For example, the electron configuration of sodium is 1s22s22p63s1.
However, the standard notation often yields lengthy electron configurations (especially for elements having a relatively large atomic number). To shorten such electron configurations, the Aufbau principle and the Pauli exclusion principle are applied. According to the Aufbau principle, electrons fill orbitals of increasing energy. According to the Pauli exclusion principle, no two electrons in the same atom can have the same set of quantum numbers.
What is the Electron Configuration of 1s22s22p63s23p3?
The electron configuration 1s22s22p63s23p3 is for the element chlorine (Cl). This configuration follows the Aufbau principle and the Pauli exclusion principle as described above.
The 1s orbital can hold two electrons, the 2s orbital can hold two electrons, and the 2p orbital can hold six electrons. The 3s orbital can hold two electrons and the 3p orbital can hold six electrons. Thus, all of the electrons in the chlorine atom are in their lowest energy state.
In summary, the electron configuration 1s22s22p63s23p3 is for the element chlorine (Cl). This configuration follows the Aufbau principle and the Pauli exclusion principle, which states that electrons fill orbitals of increasing energy and no two electrons in the same atom can have the same set of quantum numbers. This configuration describes how electrons are distributed in its atomic orbitals, which is essential for understanding the behavior of molecules.




Leave a Comment