Isotopes are a type of atom that differ from each other in the number of neutrons present in the nucleus of the atom. They are variants of elements that possess the same number of protons and electrons, but a different number of neutrons. For example, carbon-14, carbon-13, and carbon-12 are all isotopes of carbon.
In the atomic world, isotopes are very important. Since they possess the same number of protons, they have the same chemical properties. This makes them useful in a variety of applications such as medical imaging, food safety, and industrial processes.
Isotopes are divided into two categories: radioactive and stable. Radioactive isotopes are unstable and emit radiation as they decay. Stable isotopes do not emit radiation and are used in a variety of research and applications.
In this article, we will discuss the importance of isotopes, the different types of isotopes, and some examples of isotopes. We will also provide a few examples of isotopes to help us understand them better.
Atoms are the building blocks of matter and isotopes are an important part of the atomic structure. Isotopes are variants of an element that have the same proton number, but differ in their neutron number. This difference in neutron number results in different physical and chemical properties. For example, carbon-14 is radioactive while carbon-12 is stable.
Isotopes are used in a variety of applications such as medical imaging, food safety, and industrial processes. They are also used in research to study the history of the Earth and its environment.
Let’s look at some examples of isotopes.
1. Carbon-14
2. Carbon-13
3. Carbon-12
4. Oxygen-18
5. Oxygen-17
6. Uranium-238
7. Thorium-234
8. Neptunium-237
9. Plutonium-239
10. Strontium-90
These are just some of the many isotopes that can be found in nature. Each isotope has its own unique properties and applications.
Isotopes are an important part of the world around us and understanding them can help us to better understand our universe and the things in it. We hope this article has been helpful in introducing you to the world of isotopes and their various applications.
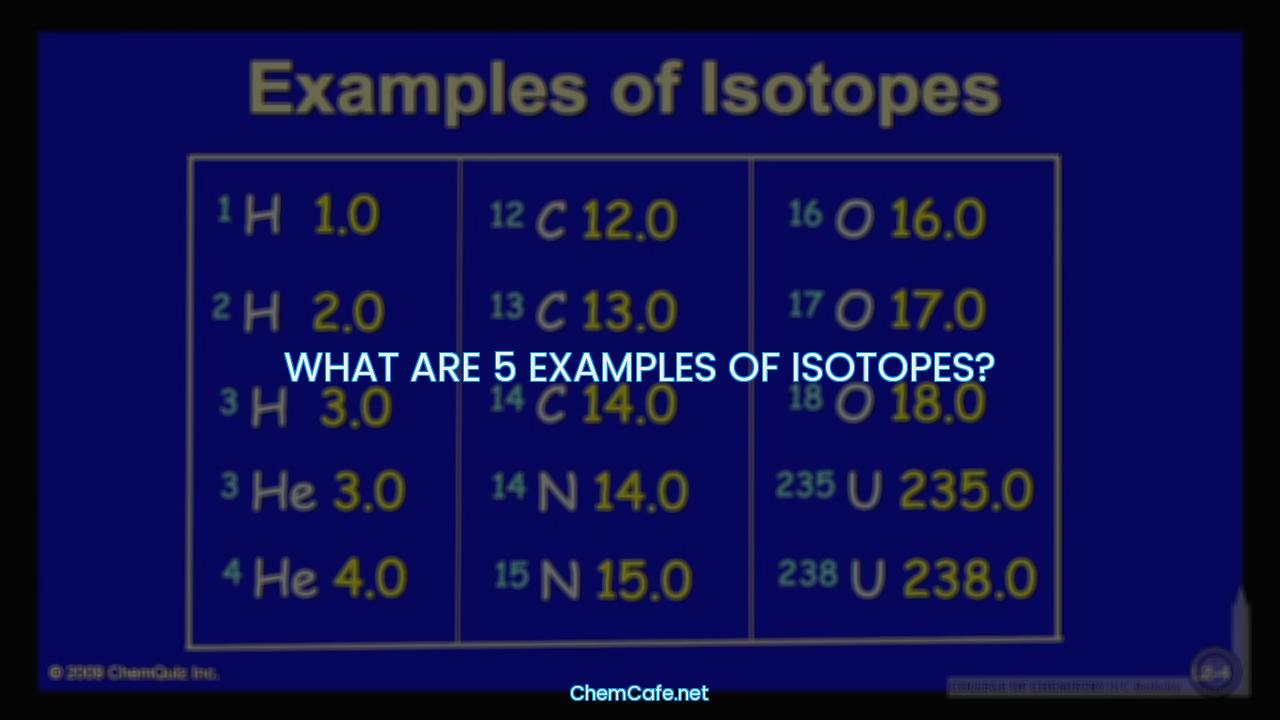
What are 5 examples of isotopes?
Isotopes are elements with the same atomic number but different mass numbers, due to the different number of neutrons they contain. Isotopes are variants of elements that differ in their nucleon number, which is the total number of protons and neutrons in their respective nuclei. Isotopes are usually found in nature, and they can be either stable or radioactive. Here are 5 examples of isotopes and their properties:
1. Carbon-12
Carbon-12 is a stable isotope of carbon, and it is the most abundant isotope of carbon found in nature. It contains 6 protons and 6 neutrons, and it is the basis for atomic mass units. Carbon-12 is an important component of the carbon cycle, and it is used in carbon dating.
2. Carbon-14
Carbon-14 is a radioactive isotope of carbon, and it is used in radiocarbon dating. It has 6 protons and 8 neutrons. Carbon-14 has a half-life of 5730 years and is produced in the upper atmosphere by the action of cosmic rays.
3. Oxygen-18
Oxygen-18 is a stable isotope of oxygen, and it is found in the Earth’s crust. It has 8 protons and 10 neutrons, and it is the most abundant oxygen isotope in nature. It is used in medical imaging and as a tracer in metabolic studies.
4. Uranium-238
Uranium-238 is a radioactive isotope of uranium, and it is the most abundant isotope of uranium found in nature. It has 92 protons and 146 neutrons, and it has a half-life of 4.5 billion years. It is used in nuclear power plants and for other industrial purposes.
5. Lead-204
Lead-204 is a stable isotope of lead, and it is one of the most abundant isotopes of lead found in nature. It has 82 protons and 122 neutrons. Lead-204 is used in the production of lead-based products and as a tracer in medical imaging.
In conclusion, isotopes are variants of elements with differing nucleon numbers. They can be either stable or radioactive, and they have various uses in scientific research and industrial applications. The 5 examples of isotopes discussed above are just a few of the many isotopes that exist in nature.
What are 10 examples of isotopes?
Isotopes are atoms of the same element that contain a different number of neutrons in the nucleus. This results in different atomic weights, which in turn, affects the characteristics of the element. Isotopes are useful for various scientific and industrial applications, such as dating rocks and tracing pollutants. Here are 10 examples of isotopes and their uses:
1. Carbon-12 and Carbon-14
Carbon-12 and carbon-14 are two carbon isotopes. Carbon-12 is stable, while carbon-14 is radioactive. Carbon-14 is used in radiocarbon dating, which is a technique used to date materials that were once living. It is also used to measure the age of fossils and artifacts.
2. Hydrogen-3 and Hydrogen-4
Hydrogen-3, also known as tritium, is a radioactive isotope of hydrogen with two neutrons in its nucleus. It is used in nuclear fusion reactions, as well as medical research. Hydrogen-4, also known as deuterium, is a stable isotope of hydrogen with one neutron in its nucleus. It is used in nuclear reactors, as well as in the manufacture of heavy water.
3. Nitrogen-14 and Nitrogen-15
Nitrogen-14 and nitrogen-15 are two nitrogen isotopes. Nitrogen-14 is a stable isotope, while nitrogen-15 is a radioactive isotope. Nitrogen-15 is used in nitrogen dating, which is a technique used to date fossilized remains. It is also used to measure the age of rocks and other geological materials.
4. Oxygen-16 and Oxygen-18
Oxygen-16 and oxygen-18 are two oxygen isotopes. Oxygen-16 is a stable isotope, while oxygen-18 is a radioactive isotope. Oxygen-18 is used in oxygen dating, which is a technique used to date geological materials such as rocks and minerals.
5. Strontium-87 and Strontium-88
Strontium-87 and strontium-88 are two strontium isotopes. Strontium-87 is a stable isotope, while strontium-88 is a radioactive isotope. Strontium-88 is used in strontium dating, which is a technique used to date geological materials such as rocks and minerals.
6. Uranium-238 and Uranium-235
Uranium-238 and uranium-235 are two uranium isotopes. Uranium-238 is a stable isotope, while uranium-235 is a radioactive isotope. Uranium-235 is used in nuclear weapons and nuclear power plants.
7. Lead-206 and Lead-207
Lead-206 and lead-207 are two lead isotopes. Lead-206 is a stable isotope, while lead-207 is a radioactive isotope. Lead-207 is used in lead dating, which is a technique used to date geological materials such as rocks and minerals.
8. Cobalt-60 and Cobalt-59
Cobalt-60 and cobalt-59 are two cobalt isotopes. Cobalt-60 is a radioactive isotope, while cobalt-59 is a stable isotope. Cobalt-60 is used in radiotherapy, which is a form of cancer treatment.
9. Iodine-131 and Iodine-129
Iodine-131 and iodine-129 are two iodine isotopes. Iodine-131 is a radioactive isotope, while iodine-129 is a stable isotope. Iodine-131 is used in diagnostic imaging, which is a form of medical imaging used to diagnose various diseases.
10. Neon-22 and Neon-21
Neon-22 and neon-21 are two neon isotopes. Neon-22 is a stable isotope, while neon-21 is a radioactive isotope. Neon-22 is used in medical imaging, which is a form of medical imaging used to diagnose various diseases.
These are just a few examples of isotopes and their uses. Isotopes have a wide variety of applications in science, industry, and medicine, and are an essential part of modern life.
What are 4 examples of isotopes?
Isotopes are variants of a chemical element that have the same number of protons but different numbers of neutrons. They can be found naturally in the Earth’s crust. In order to understand what isotopes are, it is important to have a basic understanding of the nucleus of an atom.
Atoms are composed of protons, neutrons and electrons. The protons and neutrons make up the nucleus of the atom, while the electrons orbit around it. The number of protons in an atom is what determines the element, while the number of neutrons defines the isotope.
Carbon-12 and Carbon-14
Carbon-12 and carbon-14 are two carbon isotopes. Carbon-12 is stable, while carbon-14 is radioactive. Carbon-14 is used in radiocarbon dating, which can be used to date organic materials up to 50,000 years old.
Parent and Daughter Isotopes
When a radioisotope undergoes radioactive decay, the starting isotope is called the parent isotope. Decay produces one or more daughter isotopes. For example, uranium-238 is the parent isotope that decays into the daughter isotope thorium-234.
Isotope vs Nuclide
An isotope refers to a sample of atoms. All atoms of an element have the same atomic number but may have different mass numbers due to a difference in the number of neutrons in their nuclei. A nuclide is an atom in an specific state. It can be described by its mass number, atomic number, and energy state.
Hydrogen-1, Hydrogen-2 and Hydrogen-3
Hydrogen-1 (protium), hydrogen-2 (deuterium), and hydrogen-3 (tritium) are isotopes of hydrogen. Hydrogen-1 is the most common isotope and makes up 99.985% of natural hydrogen atoms. Hydrogen-2 and hydrogen-3 are both unstable and are used in nuclear reactions.
Comparison between Isotopes and Isobars
An isotope is a variation of an element that possesses the same atomic number but a different mass number. A group of isotopes of any element will always have the same number of protons and electrons. They will differ in the number of neutrons held by their respective nuclei. An example of a group of isotopes is hydrogen-1 (protium), hydrogen-2 (deuterium), and hydrogen-3 (tritium).
Isobars are atoms that have the same mass number but different atomic numbers. They are not isotopes, but they are both atoms of different elements that have the same mass. An example of an isobar is carbon-12 and magnesium-24.
In conclusion, isotopes are atoms with the same number of protons but different number of neutrons. Carbon-12, carbon-14, uranium-238, thorium-234, hydrogen-1, hydrogen-2, and hydrogen-3 are all examples of isotopes. Isotopes are different from isobars, which are atoms with the same mass number but different atomic numbers.
What is an isotope example?
Isotopes are variants of a chemical element that have the same number of protons and electrons, but a different number of neutrons. In other words, isotopes are different forms of the same element that differ in their nucleon number. As an example, carbon-14, carbon-13, and carbon-12 are all isotopes of carbon.
Related Words
The terms related to isotopes are isotope (noun), isotopic (adjective), isotopically (adverb), and isotopy (noun).
Parent and Daughter Isotopes
When radioisotopes undergo radioactive decay, the initial isotope may be different from the resulting isotope. The initial isotope is known as the parent isotope, while the atoms produced by the reaction are called daughter isotopes. There may also be more than one type of daughter isotope produced.
As an example, when U-238 decays into Th-234, the uranium atom is the parent isotope, while the thorium atom is the daughter isotope. The atomic mass of carbon is determined by the average atomic masses of its isotopes as well as the abundance of each isotope.
Types of Isotopes
There are two main types of isotopes: radioactive isotopes and stable isotopes. Stable isotopes have a stable combination of protons and neutrons and therefore have stable nuclei. They do not undergo decay and do not pose any danger to living things, unlike radioactive isotopes.
Radioactive isotopes, on the other hand, are unstable and undergo decay. They emit radiation or particles and are extremely hazardous to living organisms. Examples of radioactive isotopes include uranium-238, strontium-90, and cesium-137.
Uses of Isotopes
Isotopes have a wide range of applications in various fields. They are used in medicine to diagnose and treat diseases, in agriculture to determine soil fertility and crop yields, and in geology to study the age of rocks and fossils. They are also used in industry to detect leaks in pipes and to measure the thickness of materials.
In addition, isotopes are used in research to study the structure of atoms, the behavior of particles, and the properties of matter. They are also used in nuclear power plants to generate electricity and in nuclear weapons to create explosions.
Isotopes are variants of chemical elements that have the same number of protons and electrons, but a different number of neutrons. There are two main types of isotopes, namely, stable isotopes and radioactive isotopes. Isotopes have a wide range of applications in various fields, including medicine, agriculture, geology, industry, research, and nuclear power.
What are 5 radioactive isotopes?
Radioactive isotopes are unstable atoms that emit radiation in order to become more stable. They are created through nuclear reactions, either naturally or artificially, and can be used for a variety of applications. Here are five of the most common radioactive isotopes and their uses:
Uranium-235 (U-235)
U-235 is a naturally occurring, fissionable isotope of uranium. It is the main fuel used in nuclear power plants, and it is also used in certain types of nuclear weapons. U-235 has a half-life of 704 million years, meaning it takes that amount of time for half of the atoms of U-235 to decay into lead.
Plutonium-239 (Pu-239)
Pu-239 is an artificially produced isotope of plutonium. It is most commonly used as a fuel in nuclear reactors, but it can also be used in nuclear weapons. Pu-239 has a half-life of 24,000 years, meaning it takes that amount of time for half of the atoms of Pu-239 to decay into uranium-235.
Carbon-14 (C-14)
C-14 is a naturally occurring isotope of carbon. It is most commonly used for radiocarbon dating, which is a technique used to determine the age of archaeological and geological samples. C-14 has a half-life of 5,730 years, meaning it takes that amount of time for half of the atoms of C-14 to decay into nitrogen-14.
Iodine-131 (I-131)
I-131 is an artificially produced isotope of iodine. It is most commonly used in nuclear medicine, as it helps to diagnose and treat certain types of thyroid cancer. I-131 has a half-life of 8 days, meaning it takes that amount of time for half of the atoms of I-131 to decay into xenon-131.
Strontium-90 (Sr-90)
Sr-90 is an artificially produced isotope of strontium. It is most commonly used in radiotherapy, as it helps to treat certain types of cancer. Sr-90 has a half-life of 28.8 years, meaning it takes that amount of time for half of the atoms of Sr-90 to decay into yttrium-90.
These five radioactive isotopes are just a small selection of the thousands of radioactive isotopes that exist. Although they all have different uses, they all have one thing in common: they are all unstable and emit radiation in order to become more stable. As such, they must be handled with extreme caution, as they can be very dangerous if not handled correctly.
What are 5 examples of stable isotopes?
Stable isotopes are chemical isotopes that are not radioactive, meaning they do not decay over time. They are found in nature and play an important role in many scientific and industrial processes. Here are five examples of stable isotopes that are commonly used today.
Hydrogen-1
Hydrogen-1, also known as protium, is the most abundant stable isotope of hydrogen. It is composed of one proton and one electron, and is found in all naturally occurring water. It is also used in the production of heavy water, which is used as a moderator in nuclear reactors.
Carbon-12
Carbon-12 is the most common isotope of carbon and makes up 99.98% of all naturally occurring carbon. It is an important component in the carbon cycle, which is the process by which carbon is exchanged between the atmosphere, biosphere, and hydrosphere. It is also used in carbon dating, a technique used to determine the age of organic material.
Oxygen-16
Oxygen-16 is the most abundant isotope of oxygen and makes up 99.76% of all naturally occurring oxygen. It is found in the atmosphere and is an important component in the water cycle, which is the process by which water is exchanged between the atmosphere, biosphere, and hydrosphere.
Nitrogen-14
Nitrogen-14 is the most abundant isotope of nitrogen and makes up 99.634% of all naturally occurring nitrogen. It is found in the atmosphere and is an important component in the nitrogen cycle, which is the process by which nitrogen is exchanged between the atmosphere, biosphere, and hydrosphere.
Lead-204
Lead-204 is the most abundant isotope of lead and makes up 1.4% of all naturally occurring lead. It is a primordial nuclide, meaning it has existed since the formation of the Solar System, and is used in a variety of industrial processes, including in the production of lead-acid batteries.
Stable isotopes play an important role in many scientific and industrial processes. These five examples are just a few of the 256 known stable isotopes of the 80 elements that have one or more stable isotopes. Understanding the properties of these isotopes can help us to better understand the processes that take place in nature.


Leave a Comment