Nucleophilicity of EtSH and MeNH2: Strong or Weak?
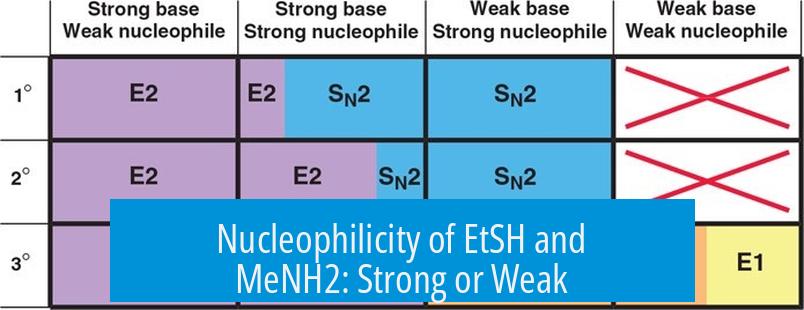
EtSH (ethylthiol) and MeNH2 (methylamine) are considered weak nucleophiles in their neutral forms. This is because their nucleophilic atoms—sulfur in EtSH and nitrogen in MeNH2—are not strongly reactive without deprotonation. The sulfide group becomes more reactive only when deprotonated, forming EtS−, a stronger nucleophile. However, as neutral molecules, both EtSH and MeNH2 participate in reactions at a slower rate.
Why Are These Nucleophiles Weak?
- Neutral EtSH retains its proton, lowering electron density on sulfur.
- Neutral MeNH2’s nitrogen lone pair is less available compared to its conjugate base.
- Neither readily donates electrons to electrophilic centers in neutral state.
Preferred Reaction Mechanisms: SN1 or SN2/E1?
Because EtSH and MeNH2 are weak nucleophiles, they favor SN1 mechanisms over SN2. The SN1 pathway proceeds via formation of a carbocation intermediate. This positively charged species reacts more readily with weak nucleophiles. Conversely, SN2 reactions require a stronger nucleophile to perform backside attack and displace the leaving group in one step, which is less efficient here.
Why Is SN1 Favored?
- The carbocation intermediate is highly electrophilic and reacts with even weak nucleophiles.
- EtSH and MeNH2 can stabilize the carbocation adduct effectively following the intermediate step.
- SN2 is less favored due to insufficient nucleophilic strength for direct displacement.
Elimination reactions (E1 or E2) are generally disfavored with EtSH and MeNH2 because elimination requires a strong base to remove a proton and form an alkene. These weak nucleophiles do not serve well as bases, reducing the likelihood of elimination pathways.
Additional Considerations: Leaving Groups
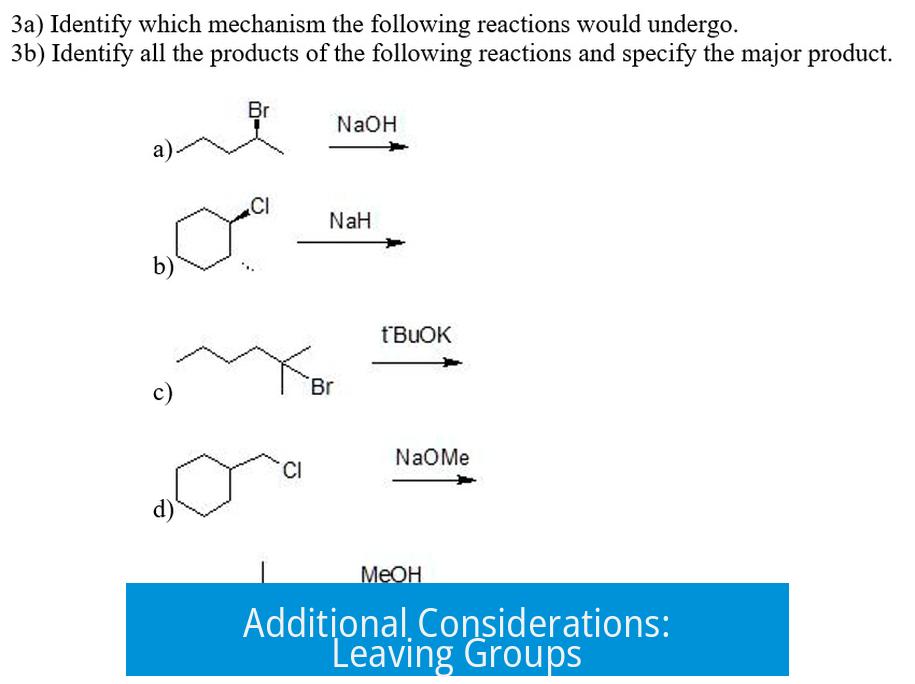
The nature of the halogen leaving group also influences reaction pathways. Better leaving groups (like iodide) more readily depart, facilitating SN1 formation of carbocations and subsequent nucleophilic attack.
| Leaving Group | Effect on Mechanism |
|---|---|
| Iodide (I−) | Excellent leaving group; favors carbocation formation and SN1 |
| Bromide (Br−) | Good leaving group; supports SN1/SN2 depending on nucleophile |
| Chloride (Cl−) | Poorer leaving group; can slow reaction or favor SN2 with strong nucleophiles |
Key Takeaways
- EtSH and MeNH2 are weak nucleophiles in neutral forms and react slowly without deprotonation.
- They favor SN1 mechanisms due to carbocation intermediate formation and weak nucleophilicity.
- SN2 is less likely because weak nucleophiles cannot efficiently perform concerted backside attack.
- Elimination reactions (E1/E2) are unlikely without stronger base conditions.
- Leaving group quality affects which pathway dominates by influencing carbocation stability.




Leave a Comment