Primer Annealing: Essential Concepts and Protocols
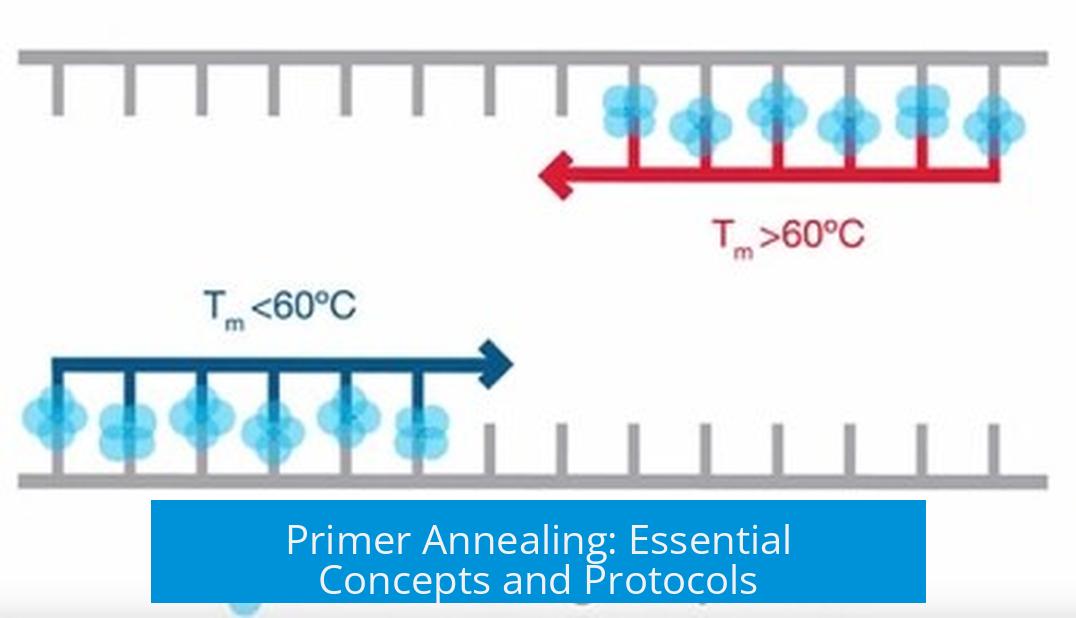
Primer annealing is the process where short DNA sequences called primers bind to complementary sequences on a DNA template. This step is crucial in molecular biology techniques such as PCR and cloning. Proper annealing ensures that primers attach specifically and efficiently to the target DNA, enabling accurate DNA amplification or cloning.
Understanding Primer Annealing
Primer annealing occurs after the DNA template is denatured—meaning its double strands are separated by heating. When the temperature is lowered to an optimal level, primers can hybridize to their complementary sequences. This temperature, known as the annealing temperature, depends on primer design and chemical conditions.
Several factors influence successful primer annealing:
- Melting temperature (Tm): Primers must have a Tm within a suitable range to ensure stable binding.
- pH and ionic strength: Maintaining a neutral pH and appropriate salt concentration promotes binding.
- Annealing temperature: It must be optimized. Too high temperatures prevent binding; too low cause non-specific binding.
Optimizing Annealing Temperature
Annealing temperatures typically range from 50°C to 65°C, depending on the primer’s Tm. If the DNA is denatured and cooled directly to 60°C in a thermocycler, primers usually anneal efficiently. However, fine-tuning the temperature can increase specificity and yield better results.
General Method for Annealing Oligonucleotides
In addition to annealing primers to DNA templates, researchers often anneal complementary oligonucleotides (short DNA strands) to form double-stranded DNA. This is especially common in cloning when creating artificial sticky ends for ligation.
A simple method involves heating a mixture of oligos to 95°C for about 10 minutes, then allowing it to cool slowly to room temperature on a benchtop. This slow cooling encourages stable and correct base pairing.
Verification of Annealing
To confirm annealing success, one can run the oligo mixture on a 2% agarose gel. Properly annealed oligos display distinct bands differing from single-stranded controls, confirming duplex formation.
Stepwise Annealing Protocol for Cloning Applications
Scientists use a refined method that combines phosphorylation and annealing for cloning oligos:
- Prepare a mixture with 3 μL of each primer, 2 μL of T4 polynucleotide kinase (PNK), and T4 ligase buffer in a 100 μL reaction volume. This adds phosphate groups essential for ligation.
- Place the tube in a 95°C heating block for 5 minutes. Use a clamp to secure the lid and prevent evaporation.
- Remove the metal block from heat safely and let it cool gradually on the bench. Slow cooling allows oligos to anneal gently and correctly.
- Dilute the annealed oligo cocktail 1:120 for use in ligation reactions with dephosphorylated vectors.
This approach reliably produces sticky ends compatible with the target vector and is well-established in molecular cloning.
Detailed Step-by-Step Annealing Oligos Protocol
This standardized protocol is effective for molecular biology workflows:
| Step | Action | Details |
|---|---|---|
| 1 | Resuspend Oligos | Prepare 100 μM stocks in Annealing Buffer. Store at -20°C. |
| 2 | Prepare Annealing Mix | Add 10 μL of each forward and reverse oligo into 80 μL of Annealing Buffer to get 10 μM final concentration. |
| 3 | Anneal Oligos | Heat mixture to 95°C, then switch off heat and cool gradually for 15 minutes in the block. |
| 4 | Cool Further | Remove the heating block and allow the tubes to cool for 45 minutes at room temperature. |
| 5 | Use or Store | Use the resulting 10 μM double-stranded oligos in ligation or store at -20°C long-term. |
Oligos engineered with sticky ends after annealing can be directly ligated into linearized vectors or digested further if needed.
Annealing Buffer Composition
Preparation of optimal buffer supports stable annealing:
- 10 mM Tris, pH 7.5
- 1 mM EDTA
- 50 mM NaCl
Recipe for 50 mL:
- 39.12 mL milli-Q water
- 400 μL 1 M Tris pH 7.5
- 80 μL 0.5 M EDTA
- 400 μL 5 M NaCl
Filter sterilize to ensure a clean solution suitable for molecular biology applications.
Summary of Key Points
- Primer annealing is binding of short DNA primers to a complementary single-stranded DNA template after denaturation.
- Annealing temperature depends on primer Tm and must be optimized for specificity.
- Oligonucleotide annealing typically involves heating to 95°C followed by gradual cooling.
- Phosphorylation before annealing enhances ligation efficiency in cloning.
- Verification of annealing can be done with agarose gel electrophoresis.
- Annealing buffer with specific ionic conditions like 10 mM Tris, 1 mM EDTA, and 50 mM NaCl supports stable duplex formation.
- The resulting annealed primers or oligos can be stored long-term at -20°C for downstream applications.
What is the optimal temperature for primer annealing to a DNA template?
Annealing usually occurs around 60°C after denaturation. However, you may need to optimize this temperature depending on your primer’s melting temperature (Tm) and sequence.
How can I verify if my oligos have annealed correctly?
Run a 2% agarose gel electrophoresis to check whether the oligos have annealed. Proper annealing will show a distinct band corresponding to double-stranded DNA.
What is a reliable method to anneal oligos for cloning?
Heat your oligo mix at 95°C for about 10 minutes, then let it cool slowly to room temperature on a bench. This gradual cooling promotes correct annealing and has been used successfully for cloning.
Why is phosphorylation important before annealing primers in cloning?
Phosphorylation adds phosphate groups to primers, enabling ligation. Using T4 polynucleotide kinase (PNK) in ligase buffer is a common step before annealing to prepare primers for cloning.
What is the composition of the annealing buffer used in primer annealing?
The buffer contains 10 mM Tris (pH 7.5), 1 mM EDTA, and 50 mM NaCl. This maintains suitable pH and ionic strength for efficient annealing.
Can annealed oligos be stored for future use? If yes, how?
Yes, after annealing, store oligos at -20°C. Proper storage preserves the double-stranded DNA integrity for long-term use.




Leave a Comment