Cloning and Subcloning: Defining Concepts and Techniques

Cloning is the process of generating an exact genetic copy of a biological entity, such as a cell, DNA segment, or organism. Subcloning, meanwhile, involves transferring a specific DNA fragment from one vector to another for further study or manipulation.
This article details the principles of cloning and subcloning, plasmid maintenance, the stability of constructs, and advances in cloning methods.
Understanding Cloning
Cloning naturally occurs when cells replicate without altering their DNA. Prokaryotes like bacteria clone themselves by binary fission, producing identical offspring.
Eukaryotic cells replicate via mitosis, making clones of themselves, except for gametes which undergo meiosis and genetic recombination.
In research, cloning also refers to duplicating biological materials such as DNA fragments or cells. Techniques like polymerase chain reaction (PCR) amplify DNA segments exponentially to assist genetic studies.
Historical Milestones in Cloning
- Early 1900s: Artificial twinning or embryo splitting was first shown by Hans Spemann on salamander embryos.
- 1952: Nuclear transfer experiments by Briggs and King cloned frog embryos using embryonic cell DNA.
- 1958: John Gurdon cloned frogs using adult intestinal cell DNA, demonstrating the potential to reprogram adult cells.
- 1970s: Recombinant DNA technology enabled transgenic clones, carrying foreign DNA segments.
- 1980s: Mammal cloning from embryonic cells began, expanding cloning into complex organisms.
- 1996: Ian Wilmut cloned Dolly the sheep via somatic cell nuclear transfer (SCNT) from differentiated adult cells.
- 2018: Successful SCNT cloning of primates (crab-eating macaques) demonstrated advanced cloning techniques in higher mammals.
Somatic Cell Nuclear Transfer (SCNT)
SCNT involves removing the nucleus from an egg cell and replacing it with the nucleus of a differentiated somatic cell. The egg cell reprograms this nucleus, allowing development into an entire organism.
This method proved adult somatic cells retain genetic information capable of reverting to embryonic pluripotency. It revolutionized cloning and contributed to therapeutic cloning and stem cell research.
Limitations and Ethical Concerns
Human cloning faces technical challenges, particularly with SCNT. Issues arise from egg cell quality affected by age and environment. Ethical debates surround cloning of embryos, especially for reproductive or research purposes.
Subcloning: Techniques and Applications
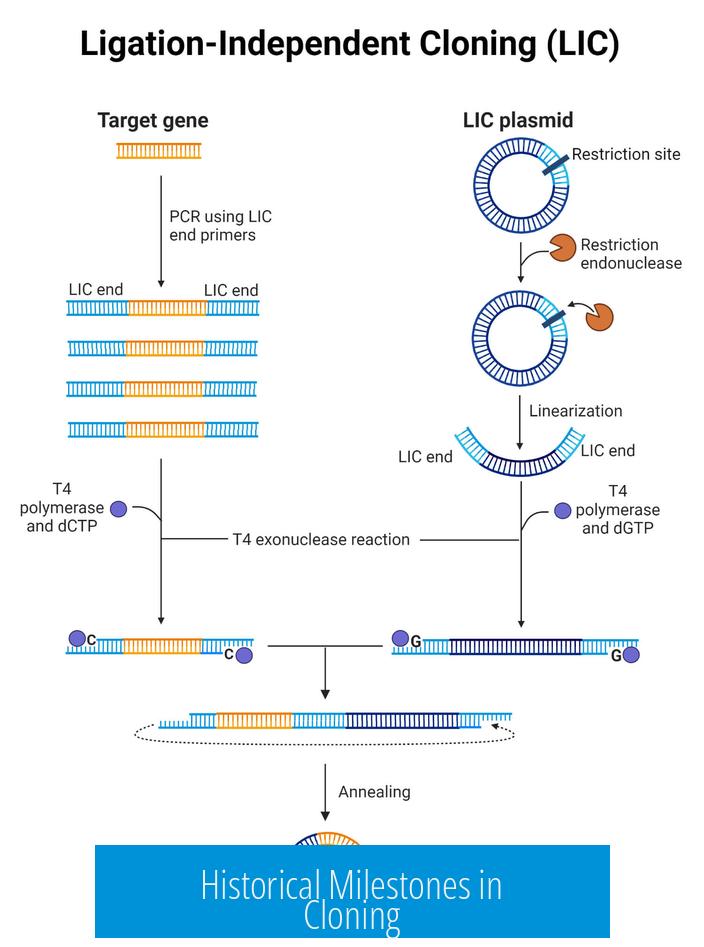
Subcloning involves extracting a specific DNA fragment from one plasmid and ligating it into another. It facilitates genetic studies, protein expression, and recombinant DNA applications.
Scientists often use subcloning to move a gene of interest into a vector compatible with a desired host organism for propagation or expression.
Resources for Learning Subcloning

Leading biotech companies such as New England Biolabs and Thermo Fisher provide free, detailed manuals covering cloning and subcloning procedures. These guides include protocols, troubleshooting tips, and technical explanations.
A recommended search method is to Google “cloning manual” alongside these company names. Their comprehensive literature aids mastery of molecular cloning techniques.
Plasmids and Their Role in Cloning and Subcloning
Structure and Replication
Plasmids are circular, extrachromosomal DNA molecules maintained independently within bacterial cells. They replicate during cell division governed by an origin of replication sequence.
Copy number, the number of plasmid copies per cell, depends mainly on the origin of replication. High-copy plasmids replicate many times per cell cycle; low-copy plasmids replicate fewer times.
Plasmid Maintenance and Stability
Antibiotic resistance genes carried on plasmids provide selective pressure for bacterial cells to retain the plasmid during division.
Larger plasmids, typically above 15 kilobases, impose a metabolic burden on host cells due to energy demands for replication. This burden can reduce plasmid stability, leading to the loss of the plasmid or rearrangement of its contents.
“Anything that slows down replication or is accompanied by a cost to fitness is generally more prone to instability.”
Recombination and Plasmid Integrity
Introduced plasmid DNA does not integrate into the bacterial chromosome in standard cloning. Laboratory *Escherichia coli* strains, such as DH5α, minimize homologous recombination to preserve plasmid integrity.
Presence of viral elements like long terminal repeats (LTRs) on plasmids increases recombination risk. To mitigate this, stable bacterial strains such as Stbl3 or modified growth conditions are employed.
Recombination must be minimized to prevent plasmid damage.
Copy Number and Plasmid Size Relationship
Generally, plasmid copy number inversely correlates with size. Larger plasmids are maintained at low copy number to reduce metabolic strain on the host.
- Small plasmids: high copy number, efficient amplification.
- Large plasmids: low copy number, improved stability.
Technical Workflow in Cloning and Subcloning
- Selection of Vector: Choose an appropriate plasmid backbone with suitable origin of replication, selectable marker, and cloning sites.
- Insertion of DNA Fragment: Use restriction enzymes or seamless cloning methods to insert desired DNA.
- Transformation: Introduce recombinant plasmid into competent bacterial cells.
- Selection: Plate cells on antibiotic-containing media to select for plasmid-containing bacteria.
- Verification: Confirm the presence and correctness of insert by colony PCR, restriction digest, or sequencing.
- Propagation: Culture positive clones to harvest plasmid DNA for downstream applications.
Common Cloning Methods
- Restriction-Ligation: Classical method involving digestion of vector and insert, then ligation.
- Gibson Assembly: Seamless cloning using overlapping DNA fragments and exonuclease/polymerase.
- TOPO Cloning: Enzyme-based insertion into specialized vectors without ligase.
Impact of Cloning and Subcloning in Biomedical Research
Cloning enables wide-ranging applications such as producing recombinant proteins, gene function studies, and genetically modified organisms.
Subcloning allows fine-tuning of genetic constructs, enabling expression under different promoters, tagging proteins, or creating mutant libraries.
Advancements in cloning techniques accelerate therapeutic development, including gene therapy and stem cell research.
Summary of Key Points
- Cloning: Creating genetically identical copies is fundamental in nature and research.
- Somatic Cell Nuclear Transfer (SCNT): Revolutionized cloning by reprogramming adult cell nuclei.
- Subcloning: Essential for moving DNA fragments between vectors for genetic analysis.
- Plasmids: Extrachromosomal DNA vectors, their origin of replication governs copy number and stability.
- Large plasmids: Less stable, maintained at low copy numbers due to metabolic costs.
- Recombination risk: Minimized by using specialized bacteria and growth conditions.
- Biotech Resources: Major companies provide detailed, free manuals aiding cloning proficiency.
- Applications: Cloning underpins biomedical research, transgenic animal creation, and therapeutic developments.
Cloning and Subcloning: Unraveling the DNA Copy Machine
What exactly are cloning and subcloning in the world of biotechnology? In simple terms, cloning is the process of creating a genetically identical copy of a cell, organism, or a piece of DNA, whereas subcloning is like the smaller, more precise sibling of cloning—transferring a DNA fragment from one vector to another to study or manipulate genes more effectively.
Sounds straightforward? Well, hold that thought because the science behind these concepts is fascinating and full of practical wizardry.
A Quick Primer on Cloning
Cloning is nature’s way of copying cells without mixing up the genetic deck. Bacteria do it all the time through binary fission, splitting into identical twins. Humans? Our skin or gut cells constantly clone themselves via mitosis. However, cloning in science doesn’t stop at cells or organisms but zooms in on DNA segments too. For instance, techniques like PCR help researchers make millions of copies of a DNA strand in a test tube—cloning at its molecular best.
Of course, cloning also stirs considerable ethical debate, especially around human embryos generated for research or reproduction. But that’s a whole other can of worms.
Subcloning: The Art of Moving DNA Around
Think of cloning as making a photocopy of a giant book (your entire genome), and subcloning is making a copy of a chapter and slipping it into a new binder. Subcloning involves taking a specific gene or DNA fragment from one plasmid—a small circular DNA molecule—and inserting it into another plasmid vector. This technique is critical for gene function studies, protein production, and creating engineered bacteria that produce useful compounds.
If you’ve ever wondered how scientists manipulate genes to build medicines or biofuels, you can thank subcloning for that precise genetic editing.
From Plasmids to Stability: The Unsung Heroes of Cloning
At the heart of cloning and subcloning lie plasmids—those tiny, extrachromosomal, circular DNA bits living inside bacterial cells. Unlike the big bacterial chromosome, plasmids can replicate independently, thanks to their origin of replication. This origin isn’t just a fancy nickname; it’s the control switch that dictates how many copies of the plasmid will exist in each bacterial cell. Copy number varies, but bigger plasmids usually keep a low profile because replicating huge DNA chunks requires energy—a metabolic burden on the bacteria.
Imagine a bacterium trying to read War and Peace instead of a postcard—it’s taxing and slows things down. This slowdown is another reason large plasmids tend to be less stable; bacteria can lose them over generations to save energy.
Why Antibiotic Resistance Genes Are Cloning’s Best Friends
One might wonder, why do plasmids carry antibiotic resistance genes? These are cloners’ secret weapons. When bacteria are grown in the presence of antibiotics, only those holding the plasmid with the resistance gene survive and multiply. This selective pressure ensures the plasmid stays safely within the bacterial population. Without it, plasmids, especially the bulky ones, might just wander off (or disappear), causing your cloning adventure to be a flop.
In the lab, this means you add antibiotics to your bacterial culture to force them to keep the plasmid—sort of like holding your favorite coffee mug hostage until you finish your drink.
Integration Versus Keeping It Separate: The DNA Drama
Here’s a critical point: the plasmid DNA introduced into bacteria doesn’t sneak into the main chromosome. It stays separate, floating freely as extrachromosomal DNA, which makes it easier to manipulate and retrieve. Bacterial lab strains, such as E. coli DH5 alpha, are genetically engineered to minimize recombination—fancy genetic swapping—that might mess up your carefully designed plasmid.
But beware viral elements like long terminal repeats (LTRs) lurking on certain plasmids—they increase the risk of unwanted DNA meddling. To outwit these troublemakers, scientists use specially chosen E. coli strains like Stbl3 that keep your plasmid stable. Adjusting growth times and temperatures can also keep the clones happy and intact.
In a nutshell, recombination can screw your plasmid and should be avoided at all cost.
Subcloning Resources: Where to Learn the Trade
If you’re new to the game or need a refresher, major biotech companies like New England Biolabs and Thermo Fisher are your best friends. They offer free, detailed cloning manuals that read better than a thrilling novel (well, for DNA nerds at least). These guides cover everything—from basic cloning tactics to product-specific protocols—and are practical goldmines.
Try Googling “cloning manual” with “New England Biolabs” or “Thermo Fisher” as your search buddies. You’ll find literature that’s some of the best reading I’ve come across on cloning topics personally.
For subcloning, similar manuals and forums exist where you can learn how to efficiently cut, ligate, and clone DNA fragments into new vectors for your experiments. Don’t hesitate to ask colleagues or look for online tutorials tailored to your specific needs.
Cloning in Action: A Brief History and Practical Impact
Cloning isn’t just a lab curiosity; it’s part of a long scientific saga. The story starts in the early 1900s, when German embryologist Hans Spemann first—through artificial “twinning”—managed to clone salamanders. He later predicted nuclear transfer, a technique realized decades later in frogs and culminating in the amazing creation of Dolly the Sheep in 1996. Dolly stunned the world by proving that DNA from a mature somatic cell could be reprogrammed to develop into an entire organism.
Fast forward to today, cloning techniques have expanded to many animals, from mice to monkeys. Even primate cloning via somatic cell nuclear transfer (SCNT) succeeded after many trials, with live crab-eating macaques born in 2018.
Cloning has revolutionized medicine, agriculture, and biological research. Therapeutic cloning paves ways for stem cell therapies. Meanwhile, subcloning fuels the production of recombinant proteins and gene therapy vectors, underpinning treatments and vaccines we rely on.
Practical Tips for Happy Cloning and Subcloning
- Choose the right plasmid vector: Select one with an appropriate origin of replication and copy number for your needs. If you want stability and high yield, balance size and metabolic burden.
- Use antibiotic selection: Always culture bacteria with antibiotics corresponding to the resistance gene on your plasmid. It keeps your clones honest!
- Prevent recombination: Use specialized strains and handle your cells gently to avoid plasmid rearrangements or loss.
- Consult trusted manuals: Use biotech company guides actively—they save time and headaches.
- Plan your subcloning strategy: Know your restriction sites, insert ends, and ligation procedures before you start. Preparation wins the cloning race.
Wrapping It Up: Cloning and Subcloning Empower Science
Cloning and subcloning are fundamental to modern molecular biology. They allow scientists to replicate and manipulate genetic material precisely, leading to breakthroughs in genetics, medicine, and biotechnology. From the humble plasmid to complex organisms, the ability to make exact copies—whether entire genomes or small DNA fragments—is a cornerstone of research.
And remember: while the science can sometimes feel like decoding the universe, tools and resources from trusted sources can make it manageable and even enjoyable. Plus, who doesn’t like playing molecular copycat now and then?
What factors affect plasmid stability during cloning?
Larger plasmids are less stable due to the high energy cost of replication. This creates a fitness burden on host bacteria, sometimes causing plasmid loss. Antibiotic resistance genes help ensure plasmids stay during cell division by providing selection pressure.
How does the origin of replication influence plasmid copy number?
The origin of replication is a DNA sequence on the plasmid. It controls how often the plasmid replicates. Larger plasmids usually have low copy numbers because they require more energy to duplicate, which strains the host cell.
Why is recombination a concern in plasmid cloning and how can it be minimized?
Recombination can disrupt plasmid DNA, especially if viral elements like LTRs are present. To reduce this, use recombination-deficient E. coli strains such as Stbl3, and adjust growing conditions like temperature and time.
What distinguishes cloning from subcloning in molecular biology?
Cloning is the process of creating identical copies of biological material, such as DNA fragments or cells. Subcloning refers specifically to transferring a DNA fragment from one vector to another, often to facilitate further manipulation or expression.
Where can I find reliable resources to learn cloning and subcloning techniques?
Major biotech companies like New England Biolabs and Thermo Fisher provide free detailed cloning manuals. Searching “cloning manual” along with these company names yields comprehensive guides that explain protocols and product use.




Leave a Comment