Is Ammonium Hydroxide Ammonia and Water, or Ammonium and Hydroxide Ions, or Both?

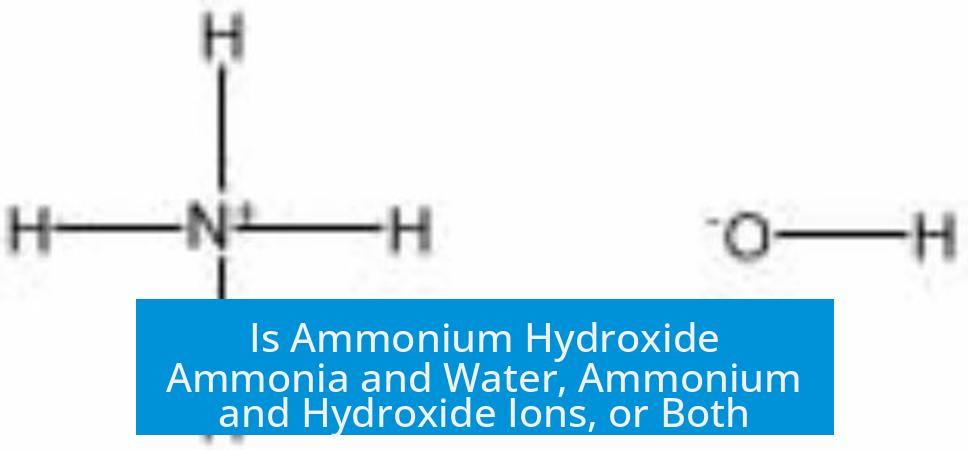
Ammonium hydroxide in aqueous solution is both ammonia dissolved in water and a mixture of ammonium (NH4+) and hydroxide (OH-) ions existing in equilibrium. It is not a single pure compound but a dynamic system involving these species.
Composition of Ammonium Hydroxide Solution
When ammonia (NH3) gas dissolves in water (H2O), most of it remains as dissolved NH3 molecules. However, some ammonia molecules accept protons (H+) from water, producing ammonium ions (NH4+) and hydroxide ions (OH-).
- This protonation reaction is partial due to ammonia’s nature as a weak base.
- The equilibrium can be represented as: NH3 + H2O ⇌ NH4+ + OH-.
Equilibrium and Relative Concentrations
This equilibrium favors the side with ammonia and water, making their concentration roughly one million times greater than the ionic species. Still, the presence of NH4+ and OH- ions imparts the solution its basic properties.
Because ammonia partially ionizes, the solution contains both molecular ammonia and separate ammonium and hydroxide ions at the same time.
Naming and Chemical Behavior
The term “ammonium hydroxide” stems from older naming conventions based on the Arrhenius concept of bases producing OH-. Since ammonia in water produces limited OH-, the older terminology applied. Today, “aqueous ammonia” is more accurate.
Production of ammonium hydroxide typically involves bubbling NH3 gas into water, resulting in this mixture of species.
Chemical Properties and pKa Values
- The ammonium ion (NH4+) has a pKa near 9-10, indicating moderate acidity.
- Water has a much higher pKa (~16), showing it is less likely to donate protons compared to ammonium.
This supports the equilibrium where ammonia tends to stay mostly un-ionized, but some fraction forms NH4+ and OH-.
Summary of Ammonium Hydroxide Composition
| Species | Role | Approximate Relative Concentration |
|---|---|---|
| NH3 (Ammonia) | Weak base, majority species | Highest (~1,000,000× more than ions) |
| H2O (Water) | Solvent/proton donor | Highest |
| NH4+ (Ammonium Ion) | Protonated ammonia, minority species | Low |
| OH- (Hydroxide Ion) | Responsible for basicity, minority species | Low |
Key Takeaways
- Ammonium hydroxide is a solution of ammonia in water involving NH3, NH4+, and OH- species in equilibrium.
- Most ammonia remains as dissolved NH3 molecules; only a small fraction ionizes to ammonium and hydroxide ions.
- The name “ammonium hydroxide” is traditional; the solution is more accurately called aqueous ammonia.
- The equilibrium between species defines the solution’s properties and basicity.
- The ion concentrations are much lower than molecular ammonia and water but are critical for the solution’s chemical behavior.
What exactly is ammonium hydroxide in water?
It is mostly ammonia (NH3) dissolved in water (H2O). Some ammonia reacts with water to form ammonium (NH4⁺) and hydroxide ions (OH⁻), but most remains as NH3 molecules.
Is ammonium hydroxide an ionic compound with NH4⁺ and OH⁻?
Ammonium hydroxide is not fully ionic. It contains some NH4⁺ and OH⁻ ions due to partial proton transfer, but it mainly exists as ammonia dissolved in water in equilibrium with those ions.
Why is ammonium hydroxide called both aqueous ammonia and ammonium hydroxide?
The name ammonium hydroxide comes from older naming based on base behavior. The correct term is aqueous ammonia since the solution is mostly NH3 in water, with some NH4⁺ and OH⁻ ions present.
How does the equilibrium between ammonia, ammonium, and hydroxide work?
Ammonia accepts a proton from water forming NH4⁺ and OH⁻ in a dynamic equilibrium. This shifts partly toward ions, but most species remain as free NH3 and H2O molecules.
Which species are present in higher concentration in ammonium hydroxide solutions?
Water and ammonia are about a million times more abundant than ammonium and hydroxide ions. The ions exist only in small amounts at equilibrium.



Leave a Comment