Understanding HSO4−: Properties and Behavior
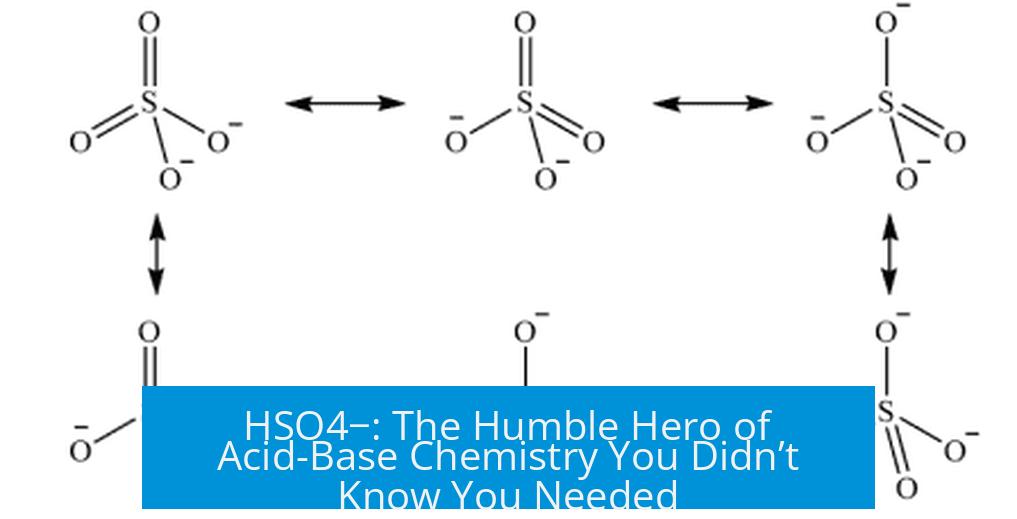
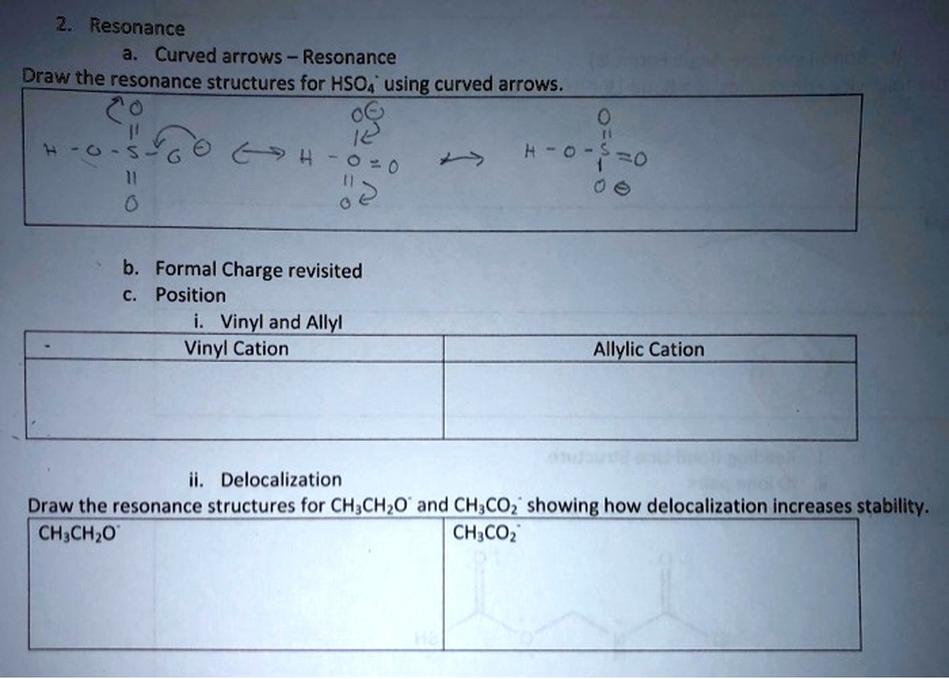
HSO4−, or the bisulfate ion, is a key intermediate species in sulfuric acid chemistry, exhibiting complex acid-base behavior and distinct equilibria in aqueous environments. This article explains its formation, equilibria, amphoteric nature, and related chemistry.
Formation and Stability in Aqueous Solutions
HSO4− forms when sulfuric acid (H2SO4) partially dissociates by losing one proton. However, it does not readily reform sulfuric acid in typical water solutions because H2SO4 is a strong acid and dissociates completely. In solutions with acids stronger than sulfuric acid, such as hydroiodic acid (HI), HSO4− can be protonated back to form sulfuric acid.
Acid-Base Equilibria Involving HSO4−
Equilibria involving HSO4− depend on the relative acid strengths present. When mixed with strong acids like nitric acid (HNO3) or sulfurous acid (H2SO3), an equilibrium exists between HSO4− and these acids, allowing partial reprotonation or dissociation. Stronger acids, such as HI, can push this equilibrium further to regenerate H2SO4.
Amphoteric Nature and pKa Values
HSO4− exhibits amphoteric behavior, able to act as an acid or base. It loses its first proton with a pKa around -3 when converting from H2SO4, indicating a highly acidic nature. The second proton dissociation, from HSO4− to sulfate ion (SO42−), has a pKa near 2. HSO4− only acts significantly as a base under extremely acidic conditions, at pH values near -2.
| Dissociation Step | Equation | pKa | Behavior |
|---|---|---|---|
| First Proton Loss | H2SO4 → HSO4− + H+ | ~ -3 | Strong acid dissociation |
| Second Proton Loss | HSO4− → SO42− + H+ | ~ 2 | Weak acid dissociation, amphoteric region |
Related Compound: Oleum
Oleum is a solution of sulfur trioxide in sulfuric acid and connects closely to HSO4− chemistry. Though not directly relevant here, oleum contains reactive species and plays a role in advanced sulfuric acid processes.
Key Takeaways
- HSO4− forms when sulfuric acid loses one proton but does not easily revert to H2SO4 in water.
- It participates in equilibria with acids of varying strengths, with reprotonation possible by stronger acids.
- HSO4− is amphoteric; its second proton dissociation has a pKa near 2.
- It acts as a base only under very acidic conditions (pH ~ -2).
- Oleum relates to sulfuric acid chemistry and involves species connected to HSO4−.
HSO4−: The Humble Hero of Acid-Base Chemistry You Didn’t Know You Needed
Ever wondered what role the bisulfate ion (HSO4−) plays in the wild world of acids and bases? You’re in the right place. This ion is much more than just a name you encounter in a chemistry textbook. It’s a dynamic player, balancing acts of proton gain and loss, stability debates, and chemical equilibria that may surprise you.
So, what exactly happens to HSO4− in aqueous solutions? The short answer is: it largely refuses to go backwards and reform sulfuric acid unless strong acid reinforcements show up. Let’s dive into why.
Why HSO4− Won’t Just Turn Back Time (or Recombine)
Imagine HSO4− as a guest who’s arrived at the party (aqueous solution) and once there, it prefers not to go back outside. When sulfuric acid (H2SO4) dissolves in water, it loses a proton and becomes this bisulfate ion. But in normal water, HSO4− won’t reform sulfuric acid. This is because sulfuric acid is such a strong acid, it immediately dissociates, like a rockstar dropping the mic and walking off stage—there’s no rewind.
However, there’s a twist. If the party suddenly gets more intense—say in the presence of an acid even stronger than H2SO4, like hydroiodic acid (HI)—HSO4− can be gently coaxed back into becoming sulfuric acid. In this environment, the bisulfate ion basically says, “Alright, I’ll put that proton back on.”
How Does HSO4− Play in the Acid-Base Equilibrium Sandbox?
HSO4− is quite the social ion. Its behavior in solution depends on the other acids around. For example, if you introduce acids like nitric acid (HNO3) or sulfurous acid (H2SO3), the equilibrium among these species dances delicately. HSO4− partakes in what chemists call an acid-base equilibrium with these acids.
Balance is key. With acids as strong or stronger than HNO3, HSO4− can hover in equilibrium without fully committing to giving or taking protons. When acids crank up the strength, like with HI, HSO4− is more likely to take a proton back, reversing the usual one-way ticket from H2SO4 to HSO4−.
The Amphoteric Personality of HSO4−: Acid? Base? Both?

Here’s where things get spicy. HSO4− is amphoteric. That means it can act as an acid or a base, depending on its surroundings. But—and this is a big but—you have to dive deep into acidic conditions to witness its basic behavior.
To put numbers to this, the first proton lost from sulfuric acid to form HSO4− has a pKa of about -3. This low pKa tells you it’s a very strong acid, shedding that proton like nobody’s business. The second proton loss—from HSO4− to form sulfate ion SO42−—has a pKa around 2. This means HSO4− is still acidic, though less dramatically so.
To actually see HSO4− act like a base—grabbing a proton instead of losing one—you’d have to lower the pH down to about -2. In real-world terms, that’s an extremely acidic environment, where even protons have trouble keeping track of who belongs where.
Oleum: The Sulfuric Acid’s Cooler Cousin
Before we pause our bisulfate deep dive, let’s throw in a fun fact: Oleum. If you’re wondering what it has to do with HSO4−, here’s a quick takeaway. Oleum is a solution of sulfur trioxide (SO3) in sulfuric acid. It can be thought of as an “acid booster” that relates to sulfuric acid chemistry. Though not strictly about HSO4−, oleum’s existence reminds us the sulfuric acid family is vast and chemically diverse, with members that can shift equilibria and species around—including our bisulfate friend.
Putting It All Together
| Aspect | Details |
|---|---|
| HSO4− Formation | Forms from dissociation of H2SO4 in water; doesn’t reform H2SO4 unless in stronger acid presence. |
| Equilibrium Behavior | Engages in acid-base equilibria with acids like HNO3 and H2SO3; stronger acids like HI can reprotonate it. |
| Acid/Base Behavior | Amphoteric but behaves mainly as acid; acts as base only at very low pH (approx. -2). |
| pKa Values | -3 for first proton loss (very strong acid); ~2 for second proton loss. |
| Related Compounds | Oleum (SO3 in H2SO4) relates to sulfuric acid chemistry but less directly to HSO4−. |
Why Should You Care About HSO4−?
It’s easy to overlook ions like HSO4−, but this bisulfate ion is vital in industrial chemistry, environmental sciences, and even protecting our planet. Sulfuric acid and its derivatives are cornerstones in fertilizer production, mineral processing, and chemical manufacturing. Understanding HSO4−’s stability and equilibrium helps chemists optimize reactions and ensures safer handling.
Plus, grasping the amphoteric nature of HSO4− is key for anyone working in acid-base equilibria, pH adjustments, or studying ocean acidification impacts, where sulfur and related compounds play roles.
Next Time You See HSO4−…
Remember, it’s a strong acid’s sidekick that won’t just go back in time, unless urged by stronger acids. It juggles equilibria like a pro and only reluctantly switches from acid to base mode under fierce acidic conditions.
Who knew this little ion had so much personality?




Leave a Comment