Understanding Water Vapor in Air

Water vapor in air is the gaseous form of water present as a component of the air mixture, distinct from visible steam. It exists invisibly, contributing to humidity and atmospheric moisture content. This vapor is part of a gas mixture that includes oxygen and nitrogen.
Distinguishing Water Vapor and Steam

Water vapor and steam are often confused but differ notably. Steam is water at a high temperature that transforms into a clear gas phase. It is invisible and can cause burns due to its heat.
Visible steam near boiling water actually consists of tiny liquid water droplets suspended in air. These droplets scatter light, making the steam visible. Vapour refers to microscopic condensate droplets mixed dynamically with air, resembling fine solids suspended in a liquid but more transient.

Physical Characteristics
- Steam: Invisible gas, can cause burns upon contact.
- Visible steam: Tiny water droplets refracting light appear as mist above boiling water.
- Water vapor: Invisible gaseous phase, part of humid air.
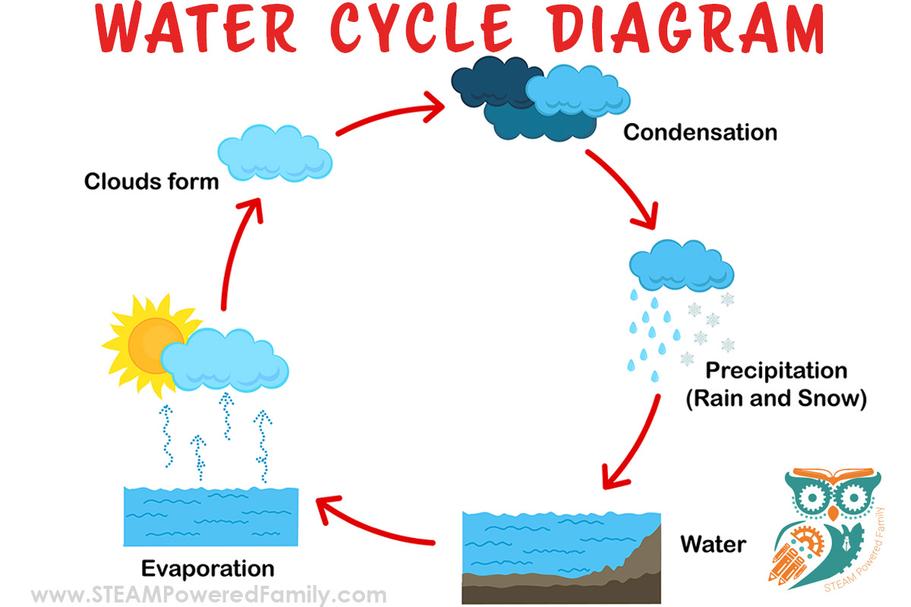
Behavior of Water Vapor and Humidity

Humidity measures the concentration of invisible water vapor in air. When air reaches saturation, water vapor condenses into liquid droplets. These droplets can remain airborne as clouds or deposit on surfaces as dew.
Such condensation occurs when the air cannot hold more vapor at a given temperature and pressure.

Quantitative Capacity of Air to Hold Water Vapor
| Temperature (°C) | Max Water Vapor (g/m3) |
|---|---|
| 0 | 4.8 |
| 10 | 9.4 |
| 20 | 17.3 |
| 30 | 30.4 |
At 20°C and standard atmospheric pressure, air can hold approximately 17 grams of water vapor per cubic meter.

Composition of Air with Water Vapor
Air is a mixture of gases, primarily nitrogen (~78%) and oxygen (~21%), with water vapor present in variable amounts. The water vapor remains in gas form under typical conditions, contributing to natural processes and climate dynamics.
Effects and Risks of Steam vs. Water Vapor
Steam, carrying substantial heat energy, can cause burns upon contact. In contrast, water vapor is simply humid air without such immediate associated risks. Understanding this distinction matters in industrial applications and safety considerations.
Key Takeaways
- Water vapor is invisible gaseous water in air, distinct from visible steam.
- Steam is high-temperature gaseous water that can be invisible and cause burns.
- Visible steam is droplets suspended in air, not pure water gas.
- Humidity measures water vapor concentration; saturation leads to droplet formation.
- At 20°C, air holds up to 17 grams water vapor per cubic meter.
- Air is a gas mixture: nitrogen, oxygen, and water vapor.
Water Vapor in Air: What It Really Is and Why You Should Care
Water vapor in air is the invisible gas form of water mixed with other gases like oxygen and nitrogen, making our atmosphere a dynamic gas solution. But what does that mean? Is water vapor the same as steam? Can you actually see it? Let’s unpack this watery mystery together and give you the lowdown on water vapor, how it behaves, and why it matters.
Clearing the Air: Water Vapor vs Steam
First up, the difference between water vapor and steam. It’s one of those subtle science facts people often mix up.
- Steam is water that’s turned into a gas at a high temperature. Picture boiling water—the steam coming off is actually invisible gas. You can’t see it, but it’s there and hot enough to burn (seriously, don’t try sticking your hand directly into boiling steam jets!).
- Water vapor is a bit different. Think of it as tiny water particles or micro-droplets suspended in the air. Imagine dust motes floating in sunlight, but these are liquid droplets mingling with gas—because water vapor dances between liquid and gas phases in the air.
When you look at the “steam” rising from your morning coffee, the billowing white cloud isn’t the steam itself, but those tiny water droplets catching the light. The real steam—the invisible gas—rises silently beyond that fluffy mist. It’s like a magic trick from Mother Nature.
How Water Vapor Behaves in Air (And What Humidity Really Means)
Ever felt that heavy, sticky feeling on a humid day? That’s water vapor making its presence felt.
Humidity measures the quantity of invisible water vapor in the air. Air can only hold so much water vapor before it gets saturated. At that point, some of the vapor condenses into tiny liquid water droplets. This is how clouds form up high and how dew forms on your car windshield in the morning.
Picture this: on a day with 100% humidity, the air is holding as much water vapor as it can. Any extra water has to find another spot—usually as tiny drops that float or settle on surfaces. These droplets are the “visible” parts we often mistake for steam.
How Much Water Can Air Actually Hold?
| Temperature (°C) | Maximum Water Vapor Capacity (g/m3) |
|---|---|
| 0 | 4.8 |
| 10 | 9.4 |
| 20 | 17.3 |
| 30 | 30.4 |
| 40 | 51.1 |
For context, at a comfortable room temperature of 20°C (68°F), the air can hold about 17 grams of invisible water vapor per cubic meter. That sounds small, but spread across every cubic meter of breathing space around you, it adds up.
So why does this matter? Well, if the temperature rises, air can chuck more water vapor inside. That’s why hot days can feel stickier and why rain clouds form when warm air full of water vapor cools down abruptly—leading to condensation and precipitation.
What’s in the Air? A Gas Cocktail With a Splash of Water
Air around us is a complex mix. Mostly nitrogen (about 78%), oxygen (about 21%), and then small amounts of other gases like argon and carbon dioxide. Water vapor is a vital part of this blend, although it varies greatly depending on weather and location.
In this gas mixture, water vapor floats just like oxygen and nitrogen. It doesn’t float like a helium balloon, but it mixes evenly, invisible and unsung.
The Good, the Bad, and the Sticky About Water Vapor
Here’s one crucial thing to remember: steam and water vapor are not the same, and they carry different risks. Steam, being hot gaseous water, can cause serious burns fast. Water vapor, or humidity, has no such immediate risk but does impact our environment, health, and comfort.
- High humidity can make the air feel clammy and uncomfortable. Ever wondered why hot and humid weather feels worse? Because our sweat doesn’t evaporate easily, making us feel hotter.
- In some cases, humidity helps keep our skin moist and airways comfortable, preventing dryness.
- Too much water vapor in the air can cause mold growth and damage to homes.
Can You See Water Vapor?
Let’s settle that once and for all — you generally ***cannot see water vapor.*** Those ghostly wisps rising from a kettle or hot shower? They’re mostly invisible steam gas cloaked in droplets of liquid water. The droplets are what catch sunlight and your eye.
Isn’t it fascinating how physical states intermingle? Tiny water droplets suspended in air work like little mirrors and prisms, reflecting and refracting light, showing up as what we casually call steam.
Practical Takeaways for Everyday Life
- Use a hygrometer to monitor indoor humidity—the ideal range is 30-50%. Too low? The air dries your skin and throat. Too high? You risk mold and discomfort.
- If you want humidity but worry about condensation, consider a humidifier with a built-in sensor to avoid saturation.
- In the kitchen, understand that the “steam” rising off boiling water signals both invisible vapor and visible droplets; be careful of burns! The invisible part can get you.
Why Should We Care About Water Vapor?
Water vapor plays a starring role in weather, climate, and comfort. It’s a tiny molecule that often flies under the radar but shapes everything from the clothes you wear to the rainfall nourishing crops.
Scientific models show water vapor acts as a greenhouse gas, trapping heat and affecting global warming more than some might realize. Simple daily experiences like humidity relate directly to this complex global system.
Final Sip of Wisdom
Next time you see steam wafting from your tea or feel a sticky summer day, remember: you’re witnessing an invisible gas—water vapor—doing its atmospheric dance. It’s a fine balance of physics and nature, keeping life comfortable, interesting, and yes, sometimes a little steamy.
So, embrace the science and keep yourself hydrated—often, the unseen is just as important as the seen.
What is the difference between water vapor and steam?
Water vapor is water in a gas form mixed with air, often invisible. Steam is water gas created at high temperatures. Visible “steam” from boiling water is actually tiny liquid droplets, not just gas.
Why can I see steam above boiling water but not steam from a pipe?
Visible steam from boiling water contains tiny liquid droplets reflecting light. Steam from a pipe is pure gas and invisible. The droplets make the steam appear white and cloudy.
How much water vapor can air hold at room temperature?
At 20°C and normal pressure, air can hold about 17 grams of invisible water vapor per cubic meter. This is the maximum before condensation starts.
What happens when air becomes saturated with water vapor?
When air reaches saturation, water vapor condenses into liquid droplets. These droplets can stay suspended as clouds or form dew on surfaces.
Is water vapor harmful like steam?
No. Water vapor in air is just humidity and does not cause burns. Steam is hot gas and can cause burns on contact.



Leave a Comment