Understanding Butyric Acid in Amateur Chemistry
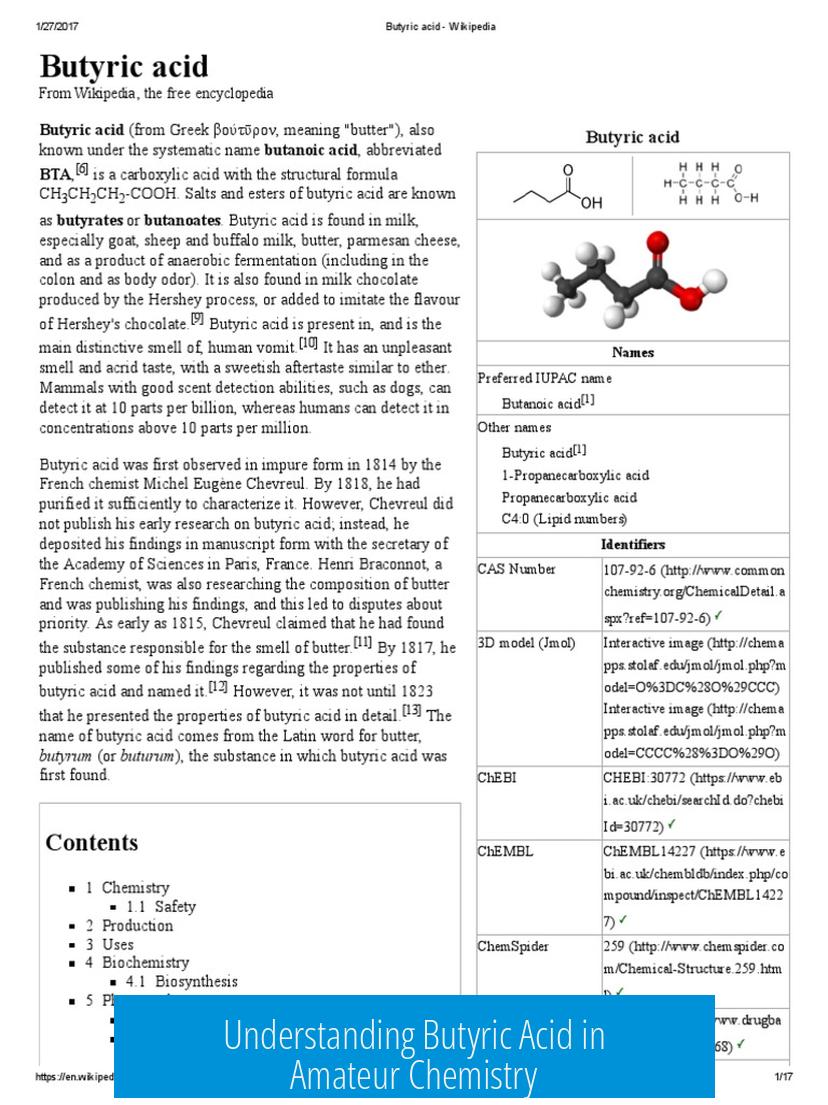
Butyric acid is a carboxylic acid known for its strong, unpleasant smell often described as rancid butter or vomit. This distinctive odor makes it notable among amateur chemists who receive it, especially by mail.
Odor Characteristics of Butyric Acid
Butyric acid emits a powerful, offensive odor that is hard to ignore. The scent resembles rancid butter or vomit and is often compared to other malodorous compounds. Because of its strong smell, it is frequently used in demonstrations or jokes, but such pranks can cause problems in storage or labs.
- Odor is a key property: rancid butter or vomit-like.
- Strong lingering smell can be a nuisance in workspaces.
- Caution needed to avoid misuse or accidental exposure.
Esters Derived from Butyric Acid
Though butyric acid itself smells foul, it is a precursor to a group of esters known as butyrates. These esters often have more pleasant, fruity odors and find uses in flavorings and fragrances.
| Compound | Smell | Notes |
|---|---|---|
| Butyric Acid | Rancid butter/vomit | Strong, unpleasant |
| Butyrate Esters | Fruity, pleasant | Degrade back to acid if handled poorly |
Improper storage can cause butyrate esters to hydrolyze back into butyric acid, releasing the strong odor again. Handling these compounds with care limits odor problems and preserves their desirable scents.
Related Chemicals and Their Odors
Other short-chain carboxylic acids have odors similar to butyric acid.
- Valeric acid shares a comparable smell with butyric acid.
- Isoamyl acetate, when concentrated, also smells unpleasant but produces a banana scent when diluted.
These examples highlight how slight changes in chemical structure or concentration influence odor perception.
Safety and Practical Considerations
Due to its powerful smell, butyric acid demands careful handling in amateur chemistry. It is prone to misuse in pranks or accidental releases. Proper storage, clear labeling, and secure containment prevent exposure to the intense odor.
- Store in airtight containers to contain odor.
- Avoid places where accidental spills or pranks may occur.
- Work in well-ventilated areas to mitigate smell issues.
Key Takeaways
- Butyric acid emits a strong, unpleasant odor similar to rancid butter or vomit.
- It forms esters with more pleasant smells but can revert to its original odor if not stored properly.
- Similar carboxylic acids, such as valeric acid, share odor characteristics.
- Safe handling and storage are critical to avoid odor nuisance and misuse.
Amateur Chemistry Just Got Real: That Stinky Bottle of Butyric Acid in the Mail

Feeling the thrill of opening a new chemistry shipment, only to be hit by a wall of stink, is a rite of passage for amateur chemists. Butyric acid—a bottle of pure “vomit smell” in liquid form—just arrived. You may love the excitement or curse the nose. Either way, let’s dive deep into what this foul-smelling compound really is, why it smells so awful, and why an amateur chemist might want to keep it on the shelf (or not!).
The Not-So-Sweet Smell of Butyric Acid
Let’s be honest—there’s nothing cute about butyric acid’s odor. It’s the infamous stench responsible for rancid butter. Imagine the smell of spoiled cream dancing with a hint of vomit—that’s your bottle right there. Some people call it “biologically offensive,” but in chemistry nerd circles, it’s somewhat a badge of honor.
Interestingly, butyric acid’s odor is so notorious, it has even inspired pranksters. Greenpeace is rumored to have unleashed it for environmental protest pranks, making its reputation for stink well-known outside labs. That tells you two things: it is potent and *definitely* not for casual sniffing!
Why Would an Amateur Chemist Want Butyric Acid? What Can You Do With It?
Despite its nose-offending nature, butyric acid isn’t just a stinky troublemaker. It’s a building block in organic chemistry, employed to create butyrate esters, which can smell surprisingly pleasant. Think ripe pineapple and sweet fruit rather than vomit. This shows the magic of chemistry: turn something foul into something delightful.
However, here’s the catch. If these esters aren’t stored properly, they degrade back into butyric acid, reviving that pukey smell all over again. So, the fun in creating fruity esters comes with a responsibility for neat storage. Not doing so equals a smelly lab (or bedroom) nightmare. Amateur chemists often learn this the hard way!
A Quick Chemistry Lesson: Esters Derived from Butyric Acid

Esters are like the perfume world’s alchemists—they can take a stink and turn it into sunshine and sweetness. Butyric acid esters provide fruity aromas used in flavorings and fragrances.
- Example: Butyl butyrate smells fruity and pineapple-like.
- Without careful storage, esters revert, releasing the dreaded butyric acid odor.
For amateurs, mastering ester synthesis can be rewarding and a good way to practice lab skills. But always remember, you’re playing with volatile chemicals—handle with care to keep pranks away and noses safe.
Meet Its Smelly Cousins: Related Chemicals
Not a fan of butyric acid’s rudeness? Let’s talk about its relatives!
Valeric acid is another stinker in the family, bearing a similar smell profile—less “vomit,” more “funky gym socks.” Isoamyl acetate, on the other hand, can smell just as offensive in high concentration, but when tamed? It’s the essence of banana candy. This teaches us something cool:
Many acids and esters can transform from wildly unpleasant smells to delightfully sweet aromas with just a pinch of chemistry magic.
Handling Butyric Acid: Keep It Safe & Away from Pranksters
If you’re thinking, “Great, I have this mysterious stinky bottle now—what could go wrong?”—let me warn you: the smell is intense and can linger for days if spilled. Worse, it’s a natural tool for pranks that nobody wants to clean up.
Amateur chemists often debate whether keeping butyric acid at home or in the lab is worth the risk. This compound is one you store securely, label clearly, and respect deeply. If you live with other people, it’s a good idea to communicate what you’re doing and ensure nobody “borrows” the bottle for olfactory mischief.
Lessons From the Foul Funk: A Personal Amateur Chemist’s Tale
One amateur chemist shared their experience: “I ordered a small bottle of butyric acid for an ester project. When the package arrived, I almost dropped it—the smell was noticeable through the packaging! My neighbors definitely found out something was up. I made a few esters, which smelled amazing… until I messed up storing one and ended up with a stinky lab. Lesson learned.”
This story highlights the practical realities of experimenting with such chemicals. It teaches the value of careful storage, good labeling, and maybe keeping a bottle of air freshener handy.
Practical Tips for Amateur Chemists Receiving Butyric Acid
- Seal It Tight: Use airtight containers to prevent leaks and strong odors escaping.
- Label Clearly: Big, bold labels keep everyone aware and avoid accidental use in a prank.
- Use Gloves and Ventilate: When handling, wear gloves and work in a well-ventilated area to reduce discomfort.
- Store Cool and Dark: This helps preserve esters made from butyric acid and limits reversion to the stink.
- Practice Ester Making: Try transforming butyric acid into pleasant-smelling compounds—chemistry’s own aroma makeover.
- Respect the Smell: Don’t insist on smelling the acid itself, unless you want a nasty surprise for your nose.
Final Thoughts: Why Embrace the Stink?
For any amateur chemist, the arrival of a bottle of butyric acid is a moment of mixed feelings—pride, excitement, and a bit of “oh no, my nose!” This humble acid teaches valuable lessons about chemical properties, experimentation, and safety. It also shows chemistry’s transformative power: turning science’s stinkiest villain into sweet-smelling heroes.
So next time you receive that stinky bottle, don’t just hide it away or complain. Embrace it. Learn from it. Make some fruity esters and prove you’re a chemist who can turn vomit smell into something delightful. Are you ready to smell the science, without holding your nose?
What causes butyric acid to have such a strong, unpleasant smell?
Butyric acid smells like rancid butter or vomit. This sharp odor is due to its chemical structure. It’s a short-chain fatty acid known for its very distinct, foul scent.
Can butyric acid be turned into pleasant-smelling compounds?
Yes, it can be converted into butyrate esters, which can smell much nicer. However, if these esters break down, they may revert to butyric acid and release the bad smell again.
Are there chemicals similar to butyric acid with less offensive odors?
Valeric acid has a similar smell to butyric acid. Isoamyl acetate also smells unpleasant when concentrated, but in diluted ester form, it smells like banana.
Why might someone be cautious about keeping butyric acid in their lab?
Its strong odor can be a problem. There’s a risk that someone might misuse it for pranks because of the smell. This is why some avoid storing it.
How should butyric acid be stored to avoid odor issues?
Keep it sealed tightly to prevent leaks. Proper storage slows ester breakdown and helps contain the smell. Avoid careless handling to minimize unwanted odors.




Leave a Comment