How Do We Know Water Always Has 2 Hydrogen and 1 Oxygen Atom?
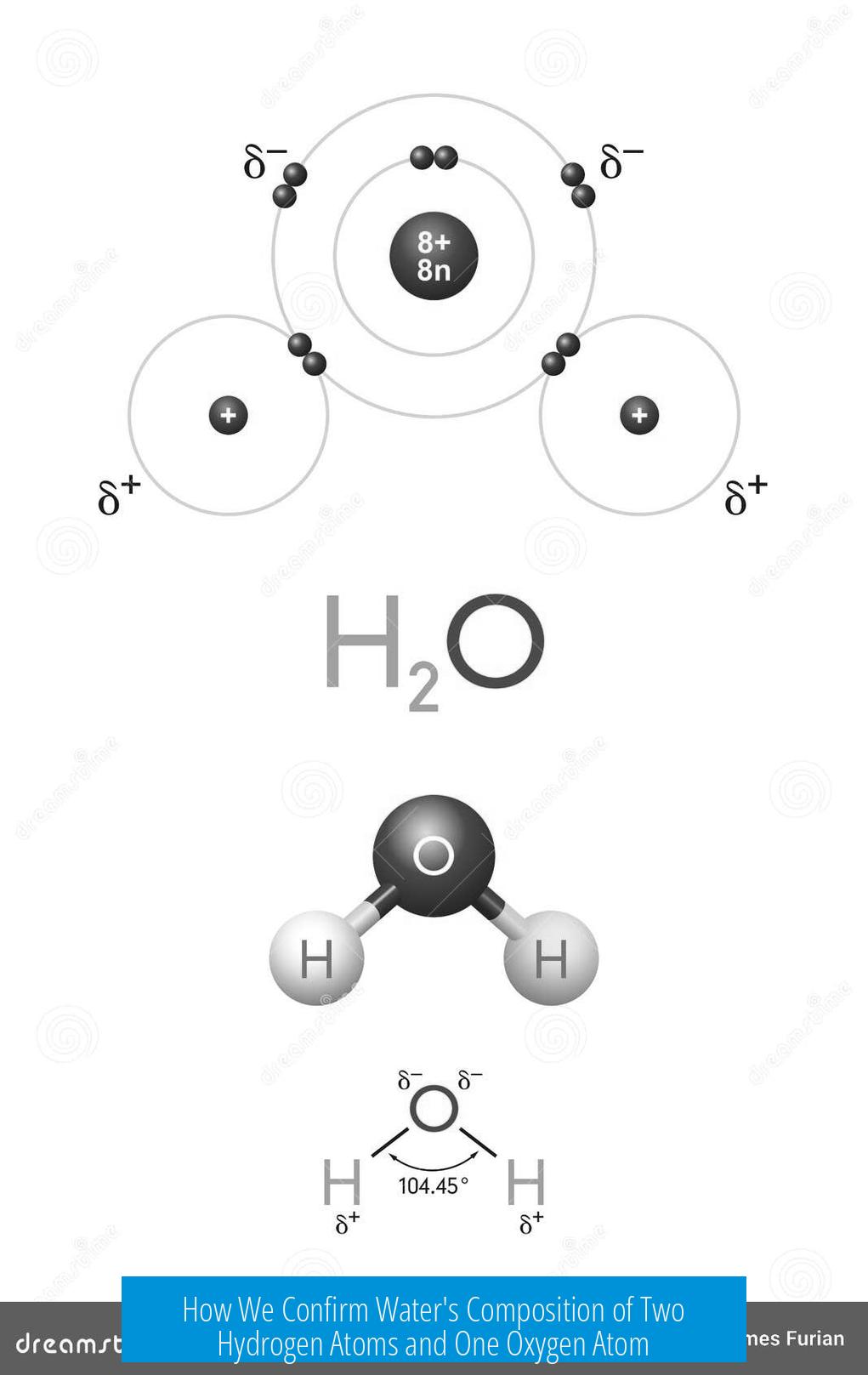
Water is defined chemically as H2O, meaning each molecule contains two hydrogen atoms and one oxygen atom. This determination comes from historical experiments, modern analytical techniques, and chemical definitions that consistently confirm this precise atomic ratio.
Historical Experimental Evidence
Combustion of Hydrogen and Oxygen Gases
Early experiments demonstrated a specific volumetric relationship between hydrogen and oxygen gases in forming water. When two volumes of hydrogen gas react with one volume of oxygen gas and ignited, they produce water without leftover gases. The reaction is:
2H2 (g) + O2 (g) → 2H2O (l)
This stoichiometry shows water forms in a ratio that equates to two hydrogen atoms per oxygen atom. The exact volume ratios reflect atoms combined in whole number ratios.
Electrolysis of Water
Splitting water into its elemental gases via electrolysis further confirms its composition. When an electric current passes through pure water, it decomposes into hydrogen and oxygen gases in a ratio of 2:1 by volume, matching the original formation ratio.
2H2O (l) → 2H2 (g) + O2 (g)
This decomposition verifies that water consists of two hydrogen atoms combined with one oxygen atom in each molecule.
Law of Definite Proportions and Atomic Ratios
Careful mass measurements showed that water always contains hydrogen and oxygen in fixed proportions by weight. This is known as the Law of Definite Proportions. Dalton’s atomic theory later translated these fixed mass ratios into atomic ratios.
For water:
- Hydrogen to oxygen mass ratio is approximately 1:8.
- Using relative atomic masses (H ≈ 1, O ≈ 16), this ratio corresponds to two hydrogens per oxygen.
These results confirmed the formula H2O, although early assumptions initially proposed different ratios.
Early Misinterpretations and Corrections
Early chemists faced challenges due to limited atomic mass data and theory. Dalton initially hypothesized water as HO, but later experiments and Mendeleev’s periodic table advancements corrected this, establishing H2O firmly. Ongoing refinement led from approximate formulas to exact molecular composition.
Modern Analytical Techniques Confirming Molecular Composition
Elemental and Molecular Analysis Methods
Modern instruments provide precise elemental ratios. Methods include:
- Inductively coupled plasma mass spectrometry (ICP-MS)
- Fourier-transform infrared spectroscopy (FTIR)
- X-ray fluorescence (XRF)
- Energy dispersive X-ray spectroscopy (EDS)
- Mass spectrometry (MS)
These techniques not only detect which elements exist in a compound but also quantify their ratios within molecules, verifying two hydrogens for every oxygen atom in water consistently.
Visualization Techniques
X-ray crystallography allows scientists to visualize atoms within molecules trapped in crystalline lattices. Water molecules have been observed directly within crystal structures, consistently showing two hydrogen atoms bonded to one oxygen.
This direct evidence transcends indirect ratios and mass calculations, providing atomic-level confirmation.
Definition and Identity of Water
Chemical Definition of Water
Water is chemically defined as H2O. Any molecule lacking this ratio is not water. If the ratio changes, the substance has different properties and is named differently. This strict definition governs chemical nomenclature and classification.
Physical and Chemical Properties Linked to Molecular Composition
The unique properties of water—such as its boiling point, surface tension, solvent abilities, and density—derive from its consistent molecular structure of two hydrogens and one oxygen. Any deviation alters these properties, supporting the definition.
Mass and Volume Consistency
Pure water has predictable mass per volume at specific temperatures, described by its molar mass (approximately 18 g/mol). This matches exactly the sum of 2 hydrogens (2 x 1 g/mol) and 1 oxygen (16 g/mol). Measuring water’s density confirms this molecular mass, reinforcing composition.
Additional Considerations
Isotopic Variations
Water molecules include hydrogen and oxygen isotopes. For example, heavy water (D2O) contains deuterium atoms (heavier hydrogen isotopes) but still maintains two hydrogens per oxygen atom. Similarly, isotopic forms like tritiated water have radioactive hydrogen isotopes.
While isotopes alter physical properties slightly, the fundamental atomic ratio remains intact. Different isotopic combinations do not change the characteristic H2O formula.
Aquatic Molecular Behavior
In liquid water, molecules form hydrogen bonds that constantly break and reform. Hydrogen and oxygen atoms can exchange protons, leading to dynamic behavior such as rapid movement of +H ions and isotope exchanges (e.g., D2O mixing to form HDO).
Despite this, each individual molecule retains two hydrogen atoms and one oxygen atom, confirming the formula on a molecular scale despite dynamic bonding networks.
Key Takeaways
- Water’s stoichiometric ratio of 2 hydrogens to 1 oxygen is confirmed through volume and mass measurements in combustion and electrolysis.
- The Law of Definite Proportions ensures consistent elemental mass ratios in water samples worldwide.
- Advances like X-ray crystallography permit direct atomic visualization, verifying the H2O structure.
- Chemical definitions restrict water to molecules with exactly this atomic ratio.
- Modern spectroscopic and mass spectrometric techniques precisely quantify elemental composition.
- Isotopes affect water’s properties but not the fundamental atomic ratio.
- Physical and chemical properties of water directly depend on this fixed molecular composition.
Why does the reaction of hydrogen and oxygen gases prove water’s formula is H₂O?
When two parts hydrogen gas react with one part oxygen gas, water forms exactly as expected. No leftover gases remain. This stoichiometric reaction confirms there are twice as many hydrogen atoms as oxygen atoms in each water molecule.
How does electrolysis confirm the 2:1 ratio of hydrogen to oxygen in water?
Electrolysis splits water into gases. It produces twice as much hydrogen gas as oxygen gas. This consistent 2-to-1 volume ratio supports that water molecules contain two hydrogens for every oxygen atom.
What role did the law of definite proportions play in identifying water’s atomic ratio?
Repeated experiments showed water’s elements always combine in fixed weight proportions. This led to the law of definite proportions, confirming water has a constant ratio of hydrogen to oxygen. Atomic theory turned these ratios into the 2:1 atom count.
How do modern techniques like X-ray crystallography confirm the composition of water?
X-ray crystallography can image atoms in crystals containing water. It reveals water molecules with two hydrogen atoms bonded to one oxygen atom. Advanced spectroscopy methods also verify elemental ratios precisely.
Why is water defined chemically as H₂O and not any other formula?
Water’s definition requires exactly two hydrogens and one oxygen atom per molecule. Different ratios create other substances, not water. Physical and chemical properties depend on this specific arrangement.
Do isotopes change water’s hydrogen-oxygen ratio?
Water can contain isotopes like deuterium or tritium, which alter mass but not the atom count. Isotopic variations don’t affect the fundamental composition of two hydrogen atoms bonded to one oxygen atom in water molecules.




Leave a Comment