How to Use pKa to Determine if a Drug is Basic or Acidic

The pKa value of a drug indicates if it is acidic or basic: if the pKa is less than 7, the drug is acidic; if it is greater than 7, it is basic. This is because pKa measures acidity on a logarithmic scale. A lower pKa means the drug more readily donates a proton, indicating acidic properties. Conversely, a higher pKa implies the drug prefers to accept protons, indicating basic characteristics.
Understanding pKa and Its Role
pKa represents the equilibrium constant for dissociation of a proton from a molecule. It determines the strength of an acid: the lower the pKa, the stronger the acid. For drugs, this means the ease with which they release or accept protons in solution affects their classification.
The strength of an acid relates inversely to the strength of its conjugate base. For instance, a molecule with a pKa of -5 is a much stronger acid than one with a pKa of 10. When the acidic proton dissociates, the resulting conjugate base’s strength depends on the original acid’s pKa.
pKa and pH Influence on Drug Protonation
The protonation state of a drug depends on both its pKa and the environmental pH. If the system’s pH is lower than the drug’s pKa, the drug tends to remain protonated (holding onto its hydrogen), behaving as an acid. If the pH is higher than the pKa, the drug loses its proton and becomes deprotonated, usually representing a basic form.
pKa Values and Basicity
Because pKa measures acidity, for basic drugs the pKa value refers to the protonated form (BH+). A high pKa in this protonated state shows a weak acidity, meaning the molecule strongly holds the proton and is a strong base. For example, bases like organolithium compounds have very high pKa values (~50), reflecting their extremely weak conjugate acids and strong basicity.
Practical Applications
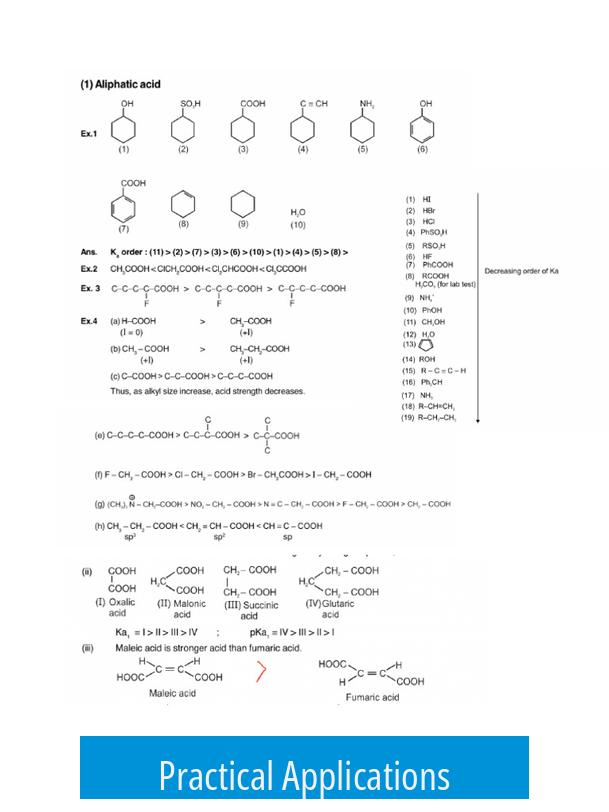
- Selective deprotonation of certain protons in a drug molecule based on different pKa values
- Using pKa for acid/base extraction techniques to separate compounds effectively
- Predicting drug solubility, absorption, and interaction based on protonation states
Key Takeaways
- pKa < 7 indicates acidic drugs; pKa > 7 indicates basic drugs.
- Lower pKa means stronger acid; higher pKa means weaker acid and stronger base (for protonated species).
- Protonation depends on pKa and environmental pH: drug protonated if pH < pKa, deprotonated if pH > pKa.
- pKa helps predict drug ionization, solubility, and behavior in biological systems.
- Extremely high pKa corresponds to very strong bases, as in organolithium compounds.
How to Use pKa to Determine if a Drug is Basic or Acidic, and What Does pKa (Strongest Basic/Acidic) Mean?
So, you want to decode the mystery of pKa to figure out if your drug is a little acidic, a bit basic, or a wild card? The key is this: pKa tells you how acidic or basic a molecule is, and helps you predict its protonation state in different environments. Let’s unravel this together with some fun facts and practical tips.
First off, pKa isn’t just a fancy number. It’s a measure on a logarithmic scale that expresses how readily a compound donates a proton. The lower the pKa, the stronger the acid. This means the molecule likes to lose its acidic proton easily, almost like a generous foodie handing out free samples.
What Does pKa Actually Mean?
Think of pKa as the molecule’s acidity meter. When a drug has a low pKa (say, below 7), it’s a strong acid. That acidic proton barely sticks around, ready to move on. The higher the pKa, especially when over 7, the more basic the drug is — unable to give up that proton easily. So, you can classify drugs like this in water:
- pKa < 7 = Acidic drug
- pKa > 7 = Basic drug
Easy, right? But here’s the twist: pKa actually measures the acidity of the protonated form of the molecule, especially important for bases. So when we talk about bases, it’s about how strong an acid their protonated form is.
Acid vs. Base: The Tug of War

Here’s a handy fact to keep in mind: the stronger the acid, the weaker its conjugate base. That means if you start with a drug having a pKa of -5, it’s a much stronger acid than one with pKa 10. And in turn, its conjugate base (the molecule once it loses the proton) is weaker. What this means is a strong acid doesn’t hold on to its proton tightly, so its partner—the base—isn’t eager to grab it back.
On the other hand, if your drug’s protonated form has a very high pKa (like 15), it’s a very weak acid. This means the base form of the drug is strong because it’s holding onto that proton tightly, refusing to give it up. Confused? Imagine a clingy kid refusing to let go of candy—that’s your strong base holding on to its proton.
How Does pKa Help Us Understand a Drug’s Behavior in the Body?
Because our body fluids have specific pH values — stomach acid is very acidic (pH ~1-2), while blood is nearly neutral (pH ~7.4) — knowing the pKa helps predict how the drug behaves in the body. If the environment’s pH is lower than the drug’s pKa, the drug stays protonated. It’s like being comfy and settled, holding onto its proton. If pH is higher than pKa, the drug tends to lose the proton (deprotonated), changing its charge and solubility.
Consider aspirin, a classic acidic drug with a pKa around 3.5. In the stomach (pH 1-2), aspirin remains mostly protonated and uncharged, which helps it cross membranes. But in the blood (pH 7.4), it deprotonates, becoming charged and more soluble. This balance is vital for absorption and distribution.
Extreme Examples: OrganoLithium and the pKa Party
Want the wildest party trick? OrganoLithium reagents have pKa values near 50! That’s astronomically high compared to typical drugs. It means their conjugate acids (alkanes) are super weak acids, and so these bases are monster strong. Such extreme pKa values show just how large the gap can be, highlighting pKa’s role beyond medicinal chemistry and into the realm of super reactive reagents.
Why Does This Matter? Practical Tips for Drug Design and Use
Understanding pKa isn’t just for the chemistry nerds. It has real-world applications:
- Drug formulation: pKa guides how drugs dissolve, absorb, and distribute in the body.
- Selective deprotonation: Chemists can selectively remove protons from complex molecules by choosing conditions around the pKa values, enabling precise modifications.
- Purifying drugs: Acid-base extraction leverages differences in pKa to separate compounds efficiently.
Want your drug to be absorbed better? Look at the pKa and pH relationship. Adjust solubility by tweaking pH to favor charged or uncharged forms. This is a fundamental trick in pharmacology.
Bringing It All Together
Let’s recap how you use pKa to determine if a drug is acidic or basic:
- Check the drug’s pKa value.
- Compare it to 7 — the pH of pure water at neutral.
- If pKa < 7, your drug is acidic; if > 7, it’s basic.
- Consider the environment pH where the drug acts to predict protonation.
- Remember: pKa refers to acidity of protonated form (especially for bases), so high pKa protonated forms mean a strong base.
Here’s a question: How might drug absorption change if you administer an acidic drug in a highly basic environment, like the small intestine? Hint: it likely affects whether the drug is ionized or not, ultimately influencing how well it crosses membranes.
In conclusion, understanding pKa lets you peek behind the scenes of drug behavior. It helps you classify, predict, and even manipulate drugs smartly. And when you master this, you’ll never see a molecule the same way again—because you’ll know exactly when it’s holding onto its proton like a stubborn mule, or eager to pass it along with grace.



Leave a Comment