Is Ethanol and Ethyl Alcohol the Same Thing?
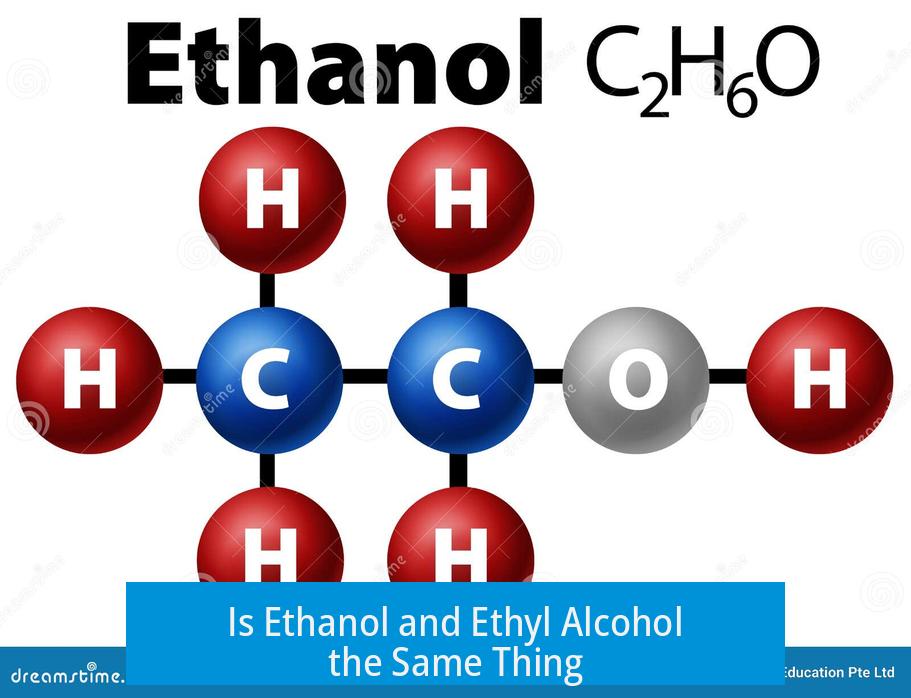
Yes, ethanol and ethyl alcohol refer to the same chemical substance. Ethanol is the systematic name given by IUPAC (International Union of Pure and Applied Chemistry), while ethyl alcohol is a common or trivial name used interchangeably in everyday contexts.
Terminology and Naming
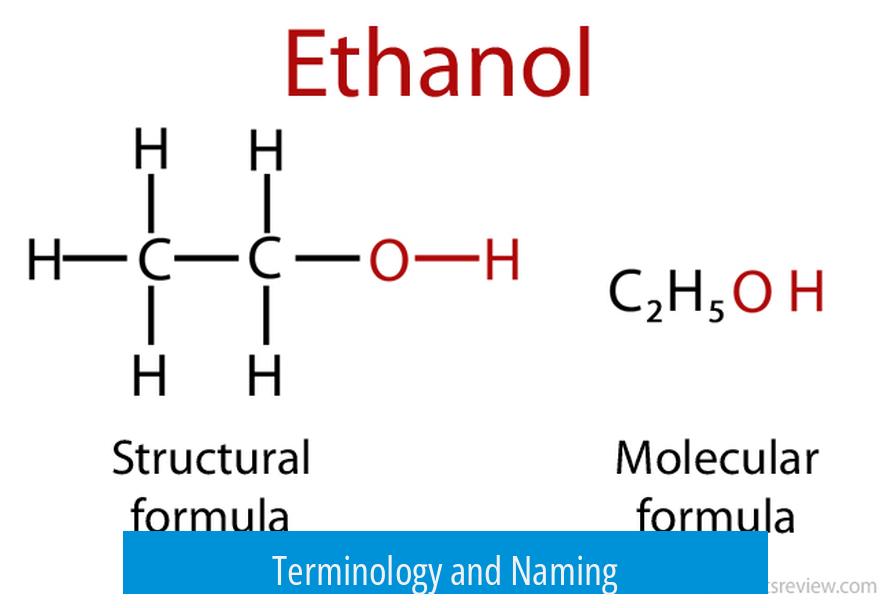
- Ethanol is the official IUPAC name. It follows standard chemical nomenclature rules.
- Ethyl alcohol is a traditional common name, widely used in industry and everyday language.
- This naming pattern extends to other alcohols:
- Methanol = methyl alcohol
- Propanol = propyl alcohol
- Butanol = butyl alcohol
Chemical Identity
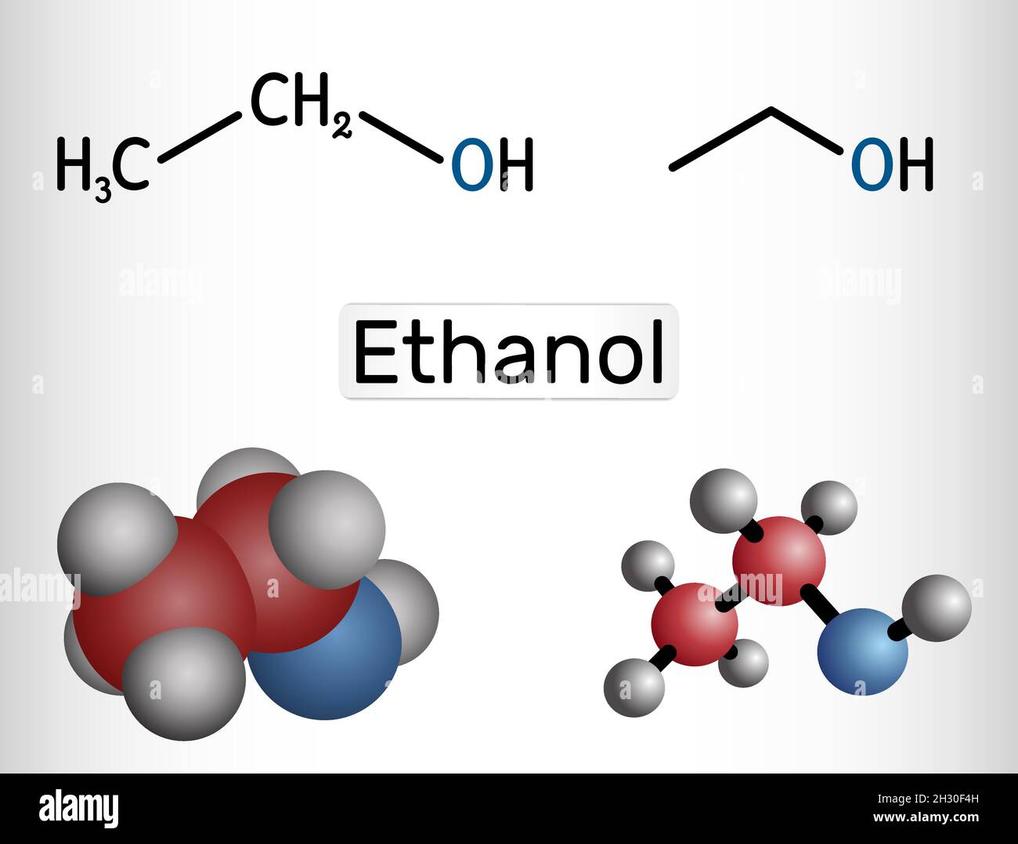
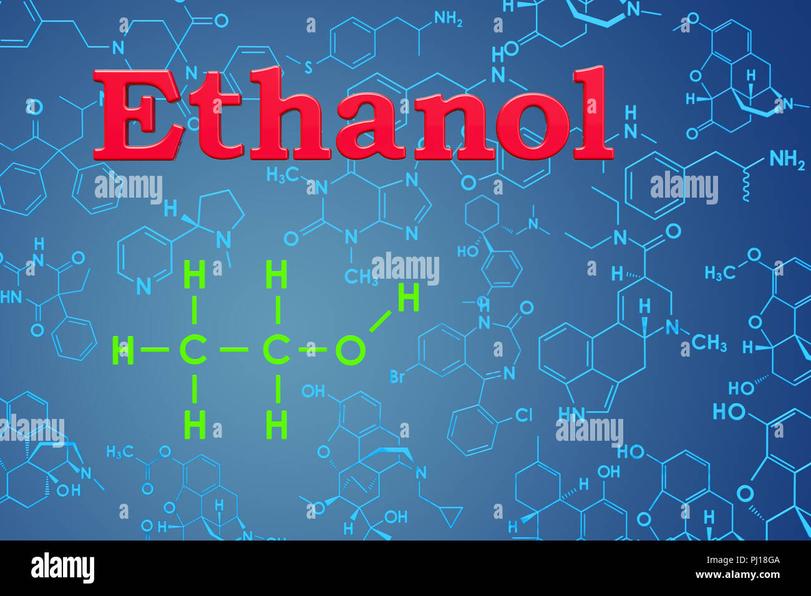
Both names denote the compound with the molecular formula C2H5OH. This formula represents an alcohol with a two-carbon chain and a hydroxyl (–OH) group attached. The terms ethanol and ethyl alcohol describe the exact same molecule.
Common Misconceptions
Sometimes, confusion arises from mixing the alcohol name “ethyl” with other chemical groups. For example, “ethyl” can be a branch or substituent in larger molecules like 3-ethylpentanoate, a different compound altogether. This does not mean ethanol and ethyl alcohol differ. Ethanol specifically refers to the two-carbon straight-chain alcohol, not just any molecule containing an ethyl group.
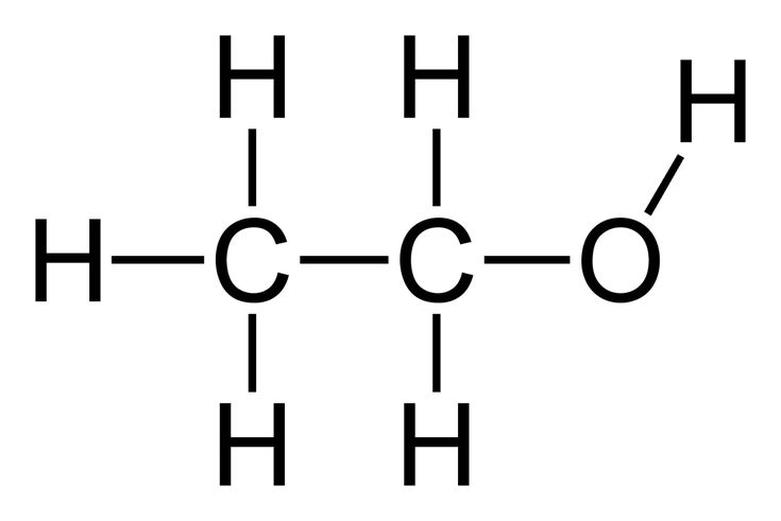
Summary of Differences
| Term | Definition | Use |
|---|---|---|
| Ethanol | IUPAC name for C2H5OH | Scientific, technical literature |
| Ethyl Alcohol | Common name for ethanol | Everyday, industrial, informal communication |
Key Takeaways
- Ethanol and ethyl alcohol are identical chemical substances.
- Ethanol is the formal IUPAC name; ethyl alcohol is a common synonym.
- Both names describe the molecule C2H5OH, a two-carbon alcohol.
- “Ethyl” alone can describe a chemical group but does not denote ethanol by itself.
- Confusion only arises when “ethyl” is used in names of larger molecules.
Is Ethanol and Ethyl Alcohol the Same Thing?
Yes, ethanol and ethyl alcohol are indeed the same compound. The difference lies mostly in naming conventions rather than the substance itself. Ethanol is the official IUPAC (International Union of Pure and Applied Chemistry) name, while ethyl alcohol is a common, everyday term people often use interchangeably.
Let’s unpack this a bit. Imagine you bump into a chemistry professor and a bartender, both talking about the liquid you find in hand sanitizers or booze. The chemist proudly calls it “ethanol,” and the bartender casually says “ethyl alcohol”—but they’re chatting about one and the same stuff. Confused already? Don’t worry, you’re not alone.
Terminology and Naming: Why Two Names for One Substance?
The naming of chemicals has long been dominated by two systems. One is the systematic system developed by IUPAC, which aims for precision and global consistency. The other is common or trivial names that have stuck around because they’re easier to say or historically entrenched.
Ethanol is the systematic name. It tells chemists exactly what to expect: a molecule made up of a two-carbon chain (that’s the “eth-” part) and an alcohol group (the “-anol”). This name comes with the precision and clarity the scientific world values.
Ethyl alcohol is a common name reflecting the same structure—“ethyl” being the prefix for the two-carbon chain and “alcohol” indicating the molecule has the OH (hydroxyl) group. This naming style is part of a pattern: methanol equals methyl alcohol, propanol equals propyl alcohol, and so forth.
This naming pattern simplifies things outside the lab but can cause headaches if you try to translate between casual talk and textbook precision. It does explain why we often say “ethyl alcohol” in everyday contexts and “ethanol” when writing scientific papers.
Same Chemical Identity: No Differences Under the Microscope
Both ethanol and ethyl alcohol share the same chemical formula: C2H5OH. What this means is they are identical molecules. Whether you call it “ethanol” or “ethyl alcohol,” the substance you’re talking about is a clear, colorless liquid.
The molecular structure involves two carbon atoms connected in a chain, with the first carbon bonded to the hydroxyl group (OH), which makes it an alcohol. This molecule is the active component not just in alcoholic beverages but also in many disinfectants and solvents.
This dual naming doesn’t imply any chemical or physical difference. It’s simply a matter of context, tradition, and audience.
Common Misconceptions About Ethyl Vs Ethanol
Here’s where things sometimes get tangled. Some people mistake “ethyl” and “ethyl alcohol” for two different things. But ethyl by itself is just an alkyl group—basically a fragment of a molecule, not an alcohol.
For example, “ethyl” shows up in other chemicals like 3-ethylpentanoate, where “ethyl” defines a branch or substituent group, not the whole molecule. Ethyl alcohol, however, always refers specifically to ethanol—when the “ethyl” group is connected to the alcohol component.
So when you hear a chemist say “ethyl alcohol,” think of it as an older, more casual name for ethanol, not something chemically distinct.
Why Does This Matter? The Practical Stuff
Understanding that ethanol and ethyl alcohol are two names for the same molecule clarifies a lot. It helps in reading labels on products, interpreting scientific texts, or just sounding smarter at parties.
If you’re buying hand sanitizer, notice the label says “ethanol 70%.” You might see “ethyl alcohol” on some older or non-scientific packaging, but it’s the same antiseptic player.
In the world of beverages like whiskey or vodka, the alcohol content is ethanol measured by volume—ethyl alcohol by its common name. This consistency ensures you’re not accidentally consuming something you didn’t intend.
A Quick Table to Clarify Naming Patterns Across Alcohols
| Systematic (IUPAC) Name | Common Name | Chemical Formula |
|---|---|---|
| Methanol | Methyl Alcohol | CH3OH |
| Ethanol | Ethyl Alcohol | C2H5OH |
| Propanol | Propyl Alcohol | C3H7OH |
| Butanol | Butyl Alcohol | C4H9OH |
Notice how each alcohol’s common name corresponds neatly with the alkyl group + alcohol format? That pattern is consistent and helps us avoid confusion.
Final Thoughts: Navigating Names with Confidence
So, is ethanol and ethyl alcohol the same thing? Absolutely. The critical takeaway is that ethanol is the official scientific name, while ethyl alcohol is a friendly nickname. Both terms denote the exact same molecule—C2H5OH.
Next time you see a chemical safety sheet or a beverage label, you can wave aside doubts. And if you want to impress someone at a dinner party, drop a line like: “You know, ethanol, or ethyl alcohol as the layman says, is the neat little molecule responsible for cheers and clean hands alike.”
Curious about how naming in chemistry might confuse other common substances? That’s a whole new rabbit hole—but for now, ethanol it is. Or is it ethyl alcohol?



Leave a Comment