When it comes to the structure of atoms, it is common knowledge that each atom has a number of electrons surrounding it. These electrons occupy different energy levels and are referred to as valence electrons. Valence electrons are the outermost electrons of an atom and determine how the atom will interact with other elements.
But why can an element only have 8 valence electrons? This question has long puzzled scientists and experts alike. Some books and dictionaries define valence electrons as “electrons in the highest principal energy level”, suggesting that an element can only have 8 valence electrons as the n−1 d orbitals fill after the n s orbitals, and then the n p orbitals fill.
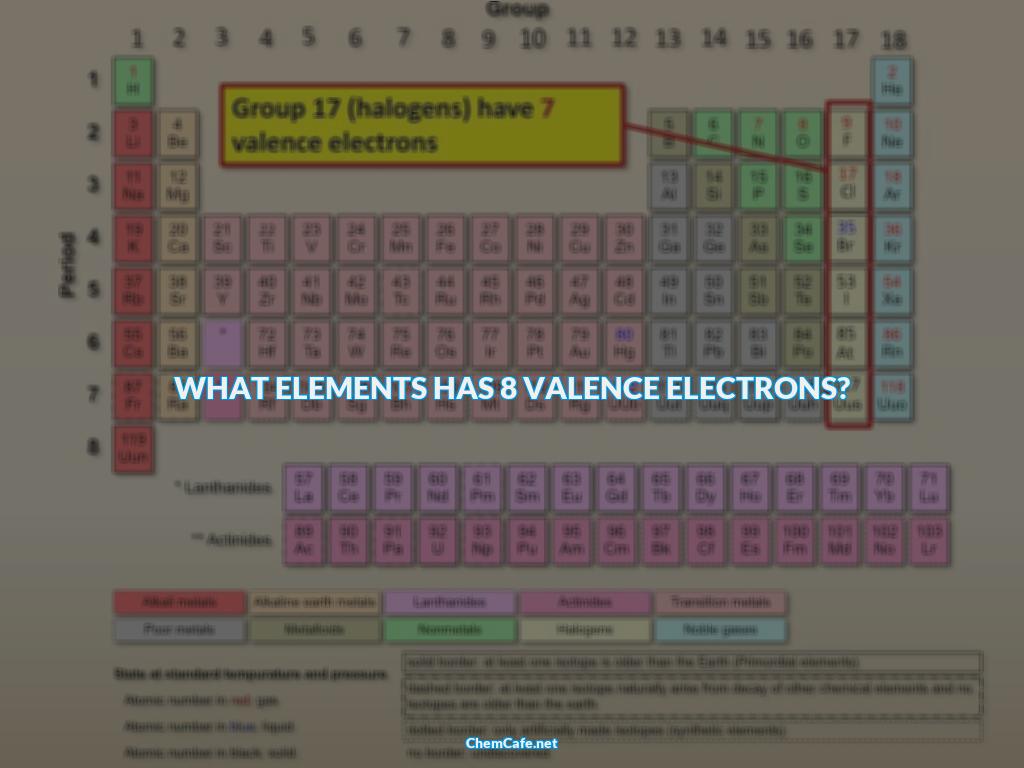
So what elements have 8 valence electrons? Any element in group 18 has eight valence electrons (except for helium, which has a total of just two electrons). Examples include neon (Ne), argon (Ar), and krypton (Kr). Interestingly, due to presence of vacant d-orbital excitation, Cl-atom can have more than eight valence electrons when it is forming hypervalent compounds like HClO4.
Valence electrons are extremely important when it comes to understanding an atom’s behavior and interaction with other elements. Knowing which elements have 8 valence electrons is also important when it comes to predicting how those elements will interact with each other. For example, sodium has 1 valence electron, while potassium has one valence electron but when it forms an ion, it has a complete octet (has eight valence electrons) in its 3rd shell.
So why is the valence shell 8? This is because it is the most stable situation for an atom. When an atom has 8 valence electrons, it is satisfied with its number of electrons, and is less likely to interact with other elements. Understanding these electrons is key to unlocking the mysteries of the universe.
Understanding the science behind valence electrons can help us understand the world around us, and help us make more informed decisions. Whether it is understanding why elements can only have 8 valence electrons, or the significance of having 8 valence electrons, understanding the science behind valence electrons can open up a whole new world of possibilities.
What elements has 8 valence electrons?
Valence electrons are the outermost electrons of an atom which are responsible for its chemical behavior. Valence electrons are also known as ‘shell electrons’ because they occupy the outermost shell of an atom. It is generally accepted that most elements can have up to eight valence electrons. This is known as the octet rule and is thought to be the most stable configuration for an atom.
Why can elements only have 8 valence electrons?
The octet rule is based on the arrangement of electrons in the shells of atoms. Electrons occupy orbitals in a specific order, with the s orbitals filling first, followed by the d orbitals. This means that the outermost shell of an atom can hold a maximum of eight electrons.
What element has a valence of 8?
Any element in group 18 of the periodic table has eight valence electrons. These elements are known as the ‘noble gases’ and include helium (He), neon (Ne), argon (Ar), krypton (Kr), and xenon (Xe).
What elements can have 8 valence electrons?
The elements that have eight valence electrons in their pure neutral forms are located in Group VIIIA of the periodic table. These elements are also known as the ‘halogens’ and include fluorine (F), chlorine (Cl), bromine (Br), iodine (I), and astatine (At).
Does potassium have 8 valence electrons?
Potassium (K) has one valence electron. This means that there is one electron in its outermost shell (4th shell). Potassium ion, on the other hand, loses an electron and has a complete octet (has eight valence electrons) in its 3rd shell.
Why is the valence shell 8?
The octet rule is based on the fact that atoms with eight valence electrons are more stable than atoms with fewer or more electrons. This is because the valence shell of an atom is the most distant from its nucleus and therefore the most difficult to hold onto. Eight electrons in the outermost shell are the most stable configuration and the most likely to be found in a neutral atom.
In summary, the valence shell of an atom is usually eight electrons in its neutral state. This is because eight is the most stable configuration for an atom and the most likely to be found in a neutral atom. Any element in group 18 has eight valence electrons, as do the elements in Group VIIIA. Potassium has one valence electron, but it can have eight when it is in its ionic form.
What are the 8 valence electrons?
The octet rule states that each atom should have 8 valence electrons to be satisfied. This rule is based on the fact that atoms strive to reach the same electron configuration as the Noble gases, which have 8 valence electrons. But how do you know if an element follows the octet rule?
Determining Valence Electrons
The first step to determining the number of valence electrons is to consider the number of lone pairs and bonds to the atom. Count the number of electrons in each of these and add them together. This will give you the number of valence electrons for the atom.
For example, chlorine (Cl) has seven electrons in its outermost shell. So we can say the Valence electron for Cl is 7.
The Octet Rule
The octet rule is that an atom will be most stable when surrounded by 8 electrons in the valence shell. An atom that does not have eight electrons will bond with other atoms to have eight electrons. A configuration that has eight electrons is also referred to as the ‘noble-gas configuration’.
Rules for Valence Electrons
Generally, elements in groups 1, 2, and 13-18 of the periodic table have eight valence electrons. Any element in group 18 has eight valence electrons (except for helium, which has a total of just two electrons). Examples include neon (Ne), argon (Ar), and krypton (Kr).
Exceptions to the Octet Rule
However, there are three general exceptions to the octet rule: Molecules, such as NO, with an odd number of electrons; Molecules in which one or more atoms possess more than eight electrons, such as SF6; and molecules in which one or more atoms possess less than eight electrons, such as BCl3.
In conclusion, the octet rule states that each atom should have 8 valence electrons to be satisfied. Generally, elements in groups 1, 2, and 13-18 of the periodic table have eight valence electrons. However, there are exceptions to the octet rule which can be found in molecules with an odd number of electrons, molecules with more than eight electrons, and molecules with less than eight electrons.
How many valence electrons are in group 18?
Valence electrons are electrons that are located in the outermost shell of an atom and are responsible for the chemical properties of that atom. Group 18 is also known as the noble gas group and all elements in this group contain eight valence electrons.
Understanding Valence Electrons
Valence electrons can be thought of as the electrons that are available for chemical bonding with other atoms. They are the electrons that are in the highest energy level (or shell) of an atom and are responsible for the chemical properties of that atom. In general, the number of valence electrons determines the type of chemical bonds that can be formed.
Group 18 Elements
Group 18 elements are called the noble gases and they all contain eight valence electrons. The noble gases are helium, neon, argon, krypton, xenon, and radon. All of these elements are non-reactive and do not form compounds or participate in chemical reactions. This is because they already have a full outer shell of eight valence electrons and thus, do not need to form bonds with other atoms.
Forming Compounds with Group 18 Elements
Despite the fact that noble gases do not usually form compounds, they can be made to do so under certain conditions. This is because noble gases can gain or lose electrons to form ions. For example, when helium is ionized, it can form a helium ion with either one or two extra electrons. This means that it is possible to form compounds with helium, neon, and other noble gases by adding or subtracting electrons.
Group 18 and the Periodic Table
Group 18 is the last vertical column of the periodic table and is often referred to as the noble gas group. All of the elements in this group have full outer shells of eight valence electrons and thus, are non-reactive. This makes them very stable and resistant to chemical reactions.
Group 18 elements all contain eight valence electrons and are thus, non-reactive and resistant to chemical reactions. Despite this, they can form compounds under certain conditions by gaining or losing electrons. Understanding the number of valence electrons in a given element can help us better understand its chemical properties and how it interacts with other elements.
Do all Group 8 elements have 8 valence electrons?
Group 8 elements are part of the periodic table, and they are categorized by their atomic number. All Group 8 elements have 8 valence electrons, but it is important to understand why this is the case. This blog section will explain the electron configuration, valence electrons, and the number of valence electrons for each group on the periodic table.
Group 8A Elements
Group 8A elements are the noble gases and all have eight electrons in the outermost energy level because their electron configuration ends in s2p6. This indicates that these elements have a full octet of eight valence electrons in their highest-energy orbitals, so they have very little tendency to gain or lose electrons to form ions, or share electrons with other elements in covalent bonds.
Group 7A Elements
Group 7A elements have seven valence electrons in their highest-energy orbitals (ns2np5). This is one electron away from having a full octet of eight electrons, so these elements tend to form anions having -1 charges, known as halides: fluoride, F-; chloride, Cl-, bromide, Br-, and iodide, I-.
Group 6A Elements
Group 6A elements have six valence electrons in their highest-energy orbitals (ns2np4). They tend to form cations with a +2 charge, known as oxides: oxygen, O2+; sulfur, S2+; selenium, Se2+; and tellurium, Te2+.
Group 9 Elements
All of the elements in group 9 have nine valence electrons. They form cations having a +3 charge, known as oxyanions: nitrate, NO3-; sulfate, SO4-2; phosphate, PO4-3; and arsenate, AsO4-3.
Valence Electrons in Each Group
Group 1 has 1 valence electron. Group 2 has 2 valence electrons. Group 13 has 3 valence electrons. Group 14 has 4 valence electrons. Group 15 has 5 valence electrons. Group 16 has 6 valence electrons. Group 17 has 7 valence electrons. Group 18 has 8 valence electrons.
In conclusion, all Group 8 elements have 8 valence electrons because their highest-energy orbitals (ns2np6) contain a full octet of eight electrons. This means that these elements have very little tendency to gain or lose electrons to form ions, or share electrons with other elements in covalent bonds. It is important to note that the number of valence electrons varies from group to group, with Group 1 having the lowest number of valence electrons, and Group 18 having the highest number.
What element has 6 valence electrons and 2 electrons?
Valence electrons are the electrons in the outermost shell, or energy level, of an atom. The number of valence electrons corresponds to an element’s group on the periodic table. All elements in the same group have the same number of valence electrons, although the number of total electrons can vary.
Group 6A elements have six valence electrons in their highest-energy orbitals (ns2np4). This means that any element in Group 6A will have six valence electrons. But the question remains: Does boron have 2 valence electrons?
Does Boron Have 2 Valence Electrons?
The answer is no. Boron has 3 valence electrons. This can be determined by looking at its group on the periodic table. Boron is in Group 13, which means that it has three valence electrons.
Do All Period 2 Elements Have 2 Electron Shells?
No. Elements in each period have the same number of electron shells as the number of that period. For example, the element sodium (Na) has 1 valence electron because it is in Group 1. This means that it has one electron shell in its highest energy orbital, which is the first shell.
Now you try. How many valence electrons does sulfur (S) have?
How Many Valence Electrons Does Sulfur Have?
Sulfur has 6 valence electrons because it is in Group 16. Remember, don’t confuse valence electrons with total electrons! Sulfur has 14 total electrons (based on its number of protons) and then 6 of these are valence electrons.
Which Element Has 6 Shells and 2 Valence Electrons?
The element with 6 shells and 2 valence electrons is oxygen. Oxygen is a second-row element of periodic table, and it has 6 shells and 2 valence electrons. Therefore, the valency of oxygen is 2.
To summarize, the element that has 6 valence electrons and 2 electron shells is oxygen. Oxygen is a second-row element of periodic table, and it has 6 shells and 2 valence electrons. Therefore, the valency of oxygen is 2.
Understanding valence electrons can help us to better understand the chemical properties of different elements. Being able to recognize the number of valence electrons within an atom can help us to predict the behavior of different elements and their possible reactions. It is important to note that all elements within a group have the same number of valence electrons, but the total number of electrons can vary.
What are two valence electrons examples?
Valence electrons are the outermost electrons of an atom and are responsible for the chemical behavior of elements. They are the electrons that form chemical bonds with other atoms. Valence electrons are the main factor in determining the chemical properties of an element.
It is important to understand the concept of valence electrons and their role in the formation of chemical bonds. Knowing the number of valence electrons can help us identify the chemical properties of an element.
In this article, we will explore the concept of valence electrons and provide two examples of valence electrons. We will also discuss how the number of valence electrons affects the chemical properties of an element.
What is a Valence Electron?
A valence electron is an electron that is located in the outermost shell of an atom. This electron is responsible for forming chemical bonds with other atoms. Valence electrons are the main factor in determining the chemical properties of an element.
The number of valence electrons in an atom can range from one to eight. Elements with fewer than four valence electrons tend to form ionic bonds, while elements with more than four valence electrons tend to form covalent bonds.
Two Valence Electrons Examples
Let’s look at two examples of valence electrons.
The first example is nitrogen. Nitrogen has five valence electrons, two in the first shell and three in the second shell. Nitrogen is an important element in many biochemical reactions and is essential for life. It forms covalent bonds with other atoms.
The second example is oxygen. Oxygen has six valence electrons, two in the first shell and four in the second shell. Oxygen is also an important element in many biochemical reactions and is essential for life. It also forms covalent bonds with other atoms.
How Do Valence Electrons Affect the Chemical Properties of an Element?
The number of valence electrons in an atom affects the chemical properties of the element. Elements with fewer than four valence electrons tend to form ionic bonds, while elements with more than four valence electrons tend to form covalent bonds.
Ionic bonds are formed when one atom gives up electrons to another atom. Covalent bonds are formed when two atoms share electrons. The type of bond formed between two atoms affects the chemical properties of the element.
For example, sodium has one valence electron and is likely to form ionic bonds with other atoms. On the other hand, carbon has four valence electrons and is likely to form covalent bonds with other atoms.
Valence electrons are the outermost electrons of an atom and are responsible for the chemical behavior of elements. They are the electrons that form chemical bonds with other atoms. Valence electrons are the main factor in determining the chemical properties of an element.
We looked at two examples of valence electrons, nitrogen and oxygen. We also discussed how the number of valence electrons affects the chemical properties of an element. Ionic bonds are formed when one atom gives up electrons to another atom and covalent bonds are formed when two atoms share electrons.
Understanding the concept of valence electrons is essential for understanding the chemical properties of elements. Knowing the number of valence electrons can help us identify the chemical properties of an element.



Leave a Comment