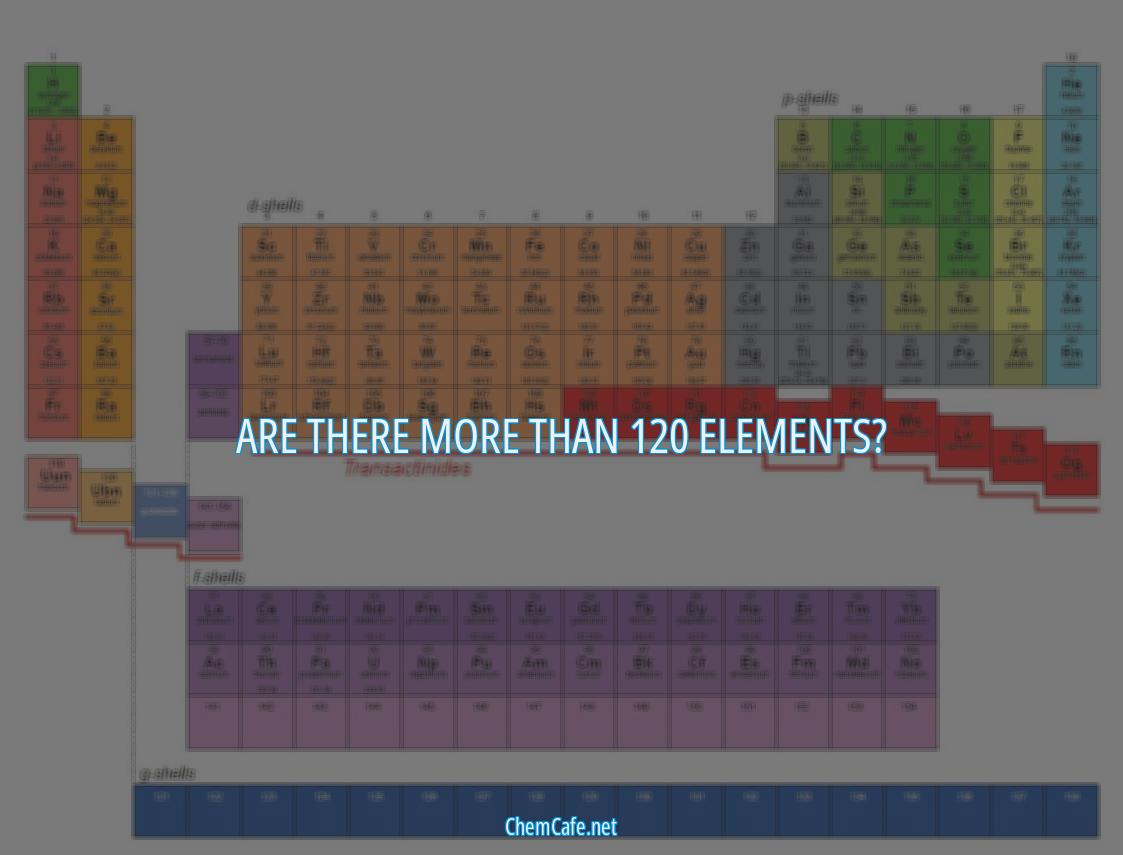
For centuries, the periodic table has been an essential tool for scientists and students alike. It’s a visual representation of the elements that make up the world around us, and it’s a crucial part of physical science. But recently, the periodic table has taken on a new life of its own.
In 2016, four new elements were added to the periodic table, completing the seventh row and bringing the total number of known elements to 118. This was a major milestone for scientists, as it marked the first time in over a century that a new row had been added to the table.
But while the addition of these new elements is certainly a cause for celebration, it also raises an interesting question: Are there more than 120 elements? The answer is yes, there are. In fact, there are nearly 120 elements that are known in the laboratory, with more being added periodically.
The key to understanding why there are more than 120 elements lies in the fact that some of the heavier elements can only be created in the laboratory. The heaviest element found in nature is element 98, or Californium, which is created from the radioactive decay of other elements. Plutonium, element 94, is the heaviest element that has ever been found that has not been the product of radioactive decay.
These heavier, man-made elements have very short half-lives, meaning that they quickly decay into lighter elements. This makes them difficult to study, as they exist only for a fraction of a second in the laboratory before decaying into something else.
For this reason, scientists continue to search for new elements, and the periodic table is growing. The recent discovery of elements 119 and beyond shows that we are entering a new era of physical science, one in which the quest to understand the world around us is never ending.
Are there more than 120 elements?
The periodic table of elements is an essential tool for scientists and students alike. It’s used to learn about the structure of the atom and the different types of elements that make up the universe. But when it comes to the number of elements, are there more than 120?
The answer is yes, there are more than 120 elements. In fact, there are currently 118 elements on the periodic table, with four new elements added in 2020. These four new elements—113, 115, 118, and 117—are located in the seventh row of the table and complete the table’s seventh row.
But even though these four new elements were discovered in the early 2000s and 2010, respectively, there is still no sign of elements 119 and beyond. So what is the heaviest element found in nature?
The heaviest element found in nature is element 98, Californium. This element is created from the radioactive decay of other elements and is not found naturally. Plutonium is another element with a higher atomic number, element 94, and is the heaviest element that has ever been found that has not been the product of radioactive decay.
In the laboratory, there have been nearly 120 elements discovered, with more being added periodically. However, the very heaviest elements are so radioactive and have such short half-lives that they have never been found in nature. These elements, like element 119, have only been made in the laboratory and have not been found in nature.
The Role of Radioactivity in Element Discovery
Radioactivity plays an important role in the discovery of new elements. Radioactive decay is a process by which the nucleus of an atom decays and emits energy in the form of particles or radiation. This process can cause the atom to transform into a different element with a higher atomic number.
For example, when uranium-238 decays, it emits an alpha particle and turns into thorium-234. This process continues until the element reaches a stable state, which is usually the heaviest element that is found in nature. This means that all elements beyond element 98 are produced in the laboratory and not found in nature.
The Future of Element Discovery
As scientists continue to explore the structure of the atom and the nature of matter, the periodic table is likely to continue to expand. However, the process of discovering new elements is a difficult one, due to the fact that the elements must be produced in the lab and have very short half-lives.
The process of element discovery is also limited by the amount of energy available to scientists. To produce elements beyond element 118, scientists will need to use intense beams of energy, such as those produced in particle accelerators. These accelerators are expensive and require a lot of energy to operate, making them difficult to use for element discovery.
Despite these challenges, scientists are continually pushing the boundaries of element discovery. As technology and energy sources continue to improve, there is a chance that new elements could be discovered in the future. Until then, the periodic table will remain at 118 elements, with element 119 and beyond still a mystery.
How many elements are there including man made?
The known universe is composed of approximately 118 chemical elements, four of which are still not officially recognized. Of these elements, only the first 98 are known to occur naturally on Earth. The remaining elements have been created synthetically, and about 32 of them occur on Earth as pure elements. The rest are found in combination in compounds.
Of the 118 elements, 80 are considered to be stable. The heaviest element found in nature is element 98, Californium, which is created from the radioactive decay of other elements. Plutonium, element 94, is the heaviest element that has ever been found that has not been produced from radioactive decay.
Elements in History
By 1900, there were 82 known elements. In 1800, there were only 30 elements and Mendeleev’s first periodic table included 28 elements. Ancient people knew about 10 elements: carbon, copper, gold, iron, lead, mercury, silver, sulfur, tin, and zinc. Arsenic, antimony, and bismuth were discovered prior to 1500 AD, while phosphorus, cobalt, and platinum were discovered by 1750.
Synthetic Elements
Technetium became the first synthetic element in 1937. Since then, scientists have been able to create more than a dozen more synthetic elements. These elements are created by bombarding atoms with particles, such as neutrons, protons, or alpha particles. The particles fuse with the atoms, forming new elements.
The Dynamic Universe
The 118 elements are only part of the story. There are more than 100 isotopes of these elements, and each element can form hundreds of compounds. In addition, elements can decay over time, creating new elements and changing the composition of the universe.
The exact number of elements, isotopes, and compounds in the universe is difficult to measure. But it’s clear that the universe is an ever-changing and dynamic place, full of infinite possibilities. With each new discovery, we learn more about the universe and its secrets.
How many elements are there?
The periodic table is a chart of elements, arranged in order of increasing atomic number. It displays the 118 elements that are currently known, ranging from hydrogen (atomic number 1) to oganesson (atomic number 118). Atoms of each element contain different numbers of protons (atomic number), as well as a unique name and element symbol.
The Elements on the Periodic Table
The 118 elements on the periodic table are divided into four categories: metals, nonmetals, metalloids, and noble gases. Metals are elements such as iron and aluminum, which are malleable and conduct electricity. Nonmetals are elements such as carbon and nitrogen, which are less malleable and do not conduct electricity. Metalloids are elements such as silicon, which have properties of both metals and nonmetals. Noble gases are elements such as helium and neon, which are inert and do not react with other elements.
The Discovery of New Elements
In 2016, four more elements were added to the periodic table, completing the seventh row. These new elements, which have atomic numbers 113, 115, 117, and 118, were discovered by scientists at the Joint Institute for Nuclear Research in Dubna, Russia, and the Lawrence Livermore National Laboratory in California. The names of these elements have yet to be officially confirmed, but they are currently referred to as ununtrium (Uut), ununpentium (Uup), ununseptium (Uus), and ununoctium (Uuo).
Elements in the Universe
How many elements are there in the known universe? Scientists estimate that there are about 118 chemical elements, but only 80 of them are stable. Many of the elements that are not stable occur in combination with other elements in compounds. The first 98 elements are known to occur naturally on Earth, while the remaining 20 have been made synthetically.
In conclusion, there are 118 elements on the periodic table. Atoms of each element contain different numbers of protons (atomic number), as well as a unique name and element symbol. The first element, with atomic number 1, is hydrogen (H). The last element is oganesson (Og), with atomic number 118. Scientists estimate that there are about 118 chemical elements in the known universe, but only 80 of them are stable. Four more elements were added to the periodic table in 2016, completing the seventh row.
Are there over 100 elements?
The answer is yes! There are 118 elements on the periodic table, ranging from hydrogen at atomic number 1 to oganesson at atomic number 118. By 1900, there were 82 known elements, and in the year 1800, there were only 30 elements. Mendeleev’s first periodic table only included 28 elements!
Ancient Elements
Ancient people knew about 10 elements: carbon, copper, gold, iron, lead, mercury, silver, sulfur, tin, and zinc. Arsenic, antimony, and bismuth were discovered prior to 1500 AD, while phosphorus, cobalt, and platinum were discovered by 1750. Most of the other natural elements were identified by 1900. Technetium became the first synthetic element in 1937. All six of these elements have been found in very small amounts in samples of uranium-rich pitchblende.
List of Elements Found in Nature
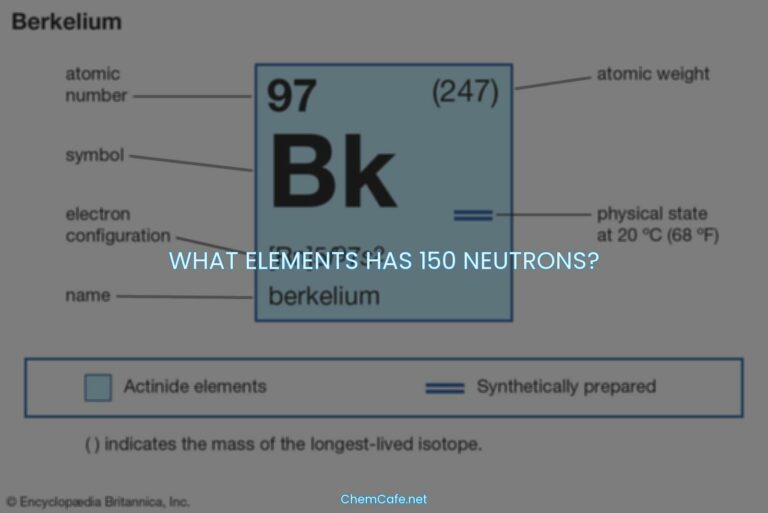
The elements found in nature are elements with atomic numbers 1 (hydrogen) through 98 (californium). Ten of these elements occur in trace amounts: technetium (No. 43), promethium (No. 61), astatine (No. 85), francium (No. 87), neptunium (No. 93), plutonium (No. 94), americium (No. 95), curium (No. 96), berkelium (No. 97), and californium (No. 98). These elements were all discovered in the 20th century.
The Discovery of New Elements
The discovery of new elements is a difficult process, as they are often present in very small amounts and difficult to separate from other elements. Scientists are constantly looking for new elements, and there may be more than the 118 currently on the periodic table.
In 2006, scientists at the Joint Institute for Nuclear Research in Dubna, Russia, announced the possible discovery of element 118, which they named oganesson. The new element was created by bombarding a lead target with calcium ions. The discovery of this element was not confirmed until 2016, and it is now officially recognized as the last element on the periodic table.
So, yes, there are over 100 elements on the periodic table. As scientific technology advances, scientists may be able to discover even more elements. For now, element 118, oganesson, is the last known element on the periodic table.
Are there only 65 known elements?
The answer to this question is no, there are now 118 known elements, though this number is constantly changing as new elements are discovered. In the past, there were only 65 known elements, and many people still believe this outdated information to be true.
The History of the Periodic Table
The periodic table, first created by Dmitri Mendeleev in 1869, originally contained 63 elements. This number increased to 65 when gallium and scandium were discovered in the late 19th century. Since then, scientific advancements have enabled the discovery of more and more elements, with the current total now standing at 118.
The Discovery of New Elements
The first 94 elements were discovered naturally, while the remaining 24 were created by humans in the laboratory. The first man-made element, technetium, was discovered in 1937 by Carlo Perrier and Emilio Segrè, while the most recently discovered element, oganesson, was synthesized in 2002 by a team led by Yuri Oganessian at the Joint Institute for Nuclear Research in Dubna, Russia.
The Naming of Elements
The 118 elements that currently make up the periodic table all have their own names and symbols, though not all of them have been universally adopted. For example, element 104 was discovered by both American and Soviet scientists, and so has two names, rutherfordium and kurchatovium, as well as two symbols, Rf and Ku.
The Future of the Table
As scientific technology continues to advance, it is likely that many more elements will be discovered in the future. Although some of these will be naturally occurring, many will be man-made and will require new names and symbols. As such, it is impossible to predict how many elements will eventually make up the periodic table, or how many more will be discovered.
In conclusion, while there were only 65 known elements in the past, today there are 118 elements, the first 94 of which occur naturally on Earth. This number is constantly increasing as new elements are discovered and named, and it is likely that this trend will continue into the future.
How many elements are there in physical science?
Physical science is an expansive field of study that encompasses a variety of disciplines, including chemistry, physics, and astronomy. It is the study of matter and energy and how they interact with each other. One of the primary components of physical science is the study of elements. But how many elements are there in physical science?
Atomic Number
At the most basic level, elements are defined by their atomic numbers. This is the number of protons in each atom, which is unique to each element. For example, hydrogen has an atomic number of 1, while helium has an atomic number of 2.
Groups of Elements
Elements can also be grouped into categories, such as alkali metals, transition metals, nonmetals, halogens, alkali earth metals, actinides, and lanthanides. Alkali metals, for example, have just 1 electron in the outer shell of their atom and are very reactive. Examples of alkali metals include lithium, sodium, and potassium.
Periodic Table
One of the most important tools for studying elements is the periodic table. This is a chart that arranges all known elements according to their atomic number, element symbol, and name. On the periodic table, each element is represented by a single box. Hydrogen is the first element, and it is located in the upper left corner of the table. The last element is oganesson, which is located in the lower right corner of the table.
How Many Elements Are There?
So, how many elements are there in physical science? The answer is that there are currently 118 elements on the periodic table, ranging from hydrogen at atomic number 1 to oganesson at atomic number 118. This number is constantly changing, however, as new elements are discovered and added to the table.
In conclusion, physical science is a vast field of study, and elements are one of the primary components. There are currently 118 elements on the periodic table, ranging from hydrogen at atomic number 1 to oganesson at atomic number 118. Each element has a unique atomic number, name, and symbol, and they can be grouped into various categories, such as alkali metals, transition metals, nonmetals, halogens, alkali earth metals, actinides, and lanthanides.


Leave a Comment