Benzene: Differences in Carbon-Carbon Bond Lengths and Their Causes
Carbon-carbon single and double bonds have different lengths because of the variation in electron density between the bonded atoms. A single bond, with two electrons, is longer due to lower attractive forces between nuclei, while a double bond, with four electrons, is shorter as the nuclei experience greater mutual attraction. In benzene, the bonds exhibit intermediate lengths because electron delocalization distributes bonding electrons evenly across the ring, resulting in bonds with partial double-bond character and lengths between those of single and double bonds.
Forces Influencing Bond Length
Bond length arises from a balance of forces:
- Repulsion between positively charged atomic nuclei pushes them apart.
- Attraction between nuclei and shared bonding electrons pulls them together.
The distance where these forces reach equilibrium defines bond length. More bonding electrons increase attraction, generally shortening the bond.
Why Single and Double Bonds Differ in Length
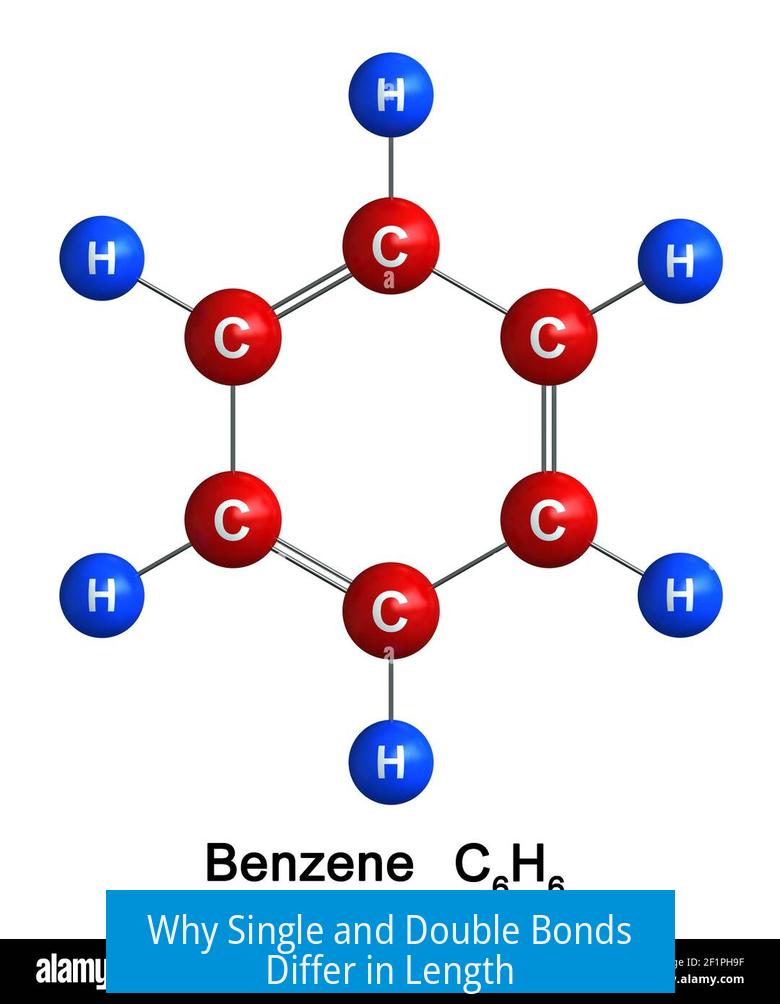
Single bonds contain two electrons in the bonding region, resulting in a certain force of attraction and thus a characteristic length.
Double bonds have four electrons situated between the nuclei. The increased negative charge strengthens the electrostatic pull, drawing atoms closer and shortening the bond.
| Bond Type | Electrons in Bond | Relative Bond Length |
|---|---|---|
| Single Bond (C–C) | 2 | Longest |
| Double Bond (C=C) | 4 | Shortest |
Benzene’s Intermediate Carbon-Carbon Bond Lengths
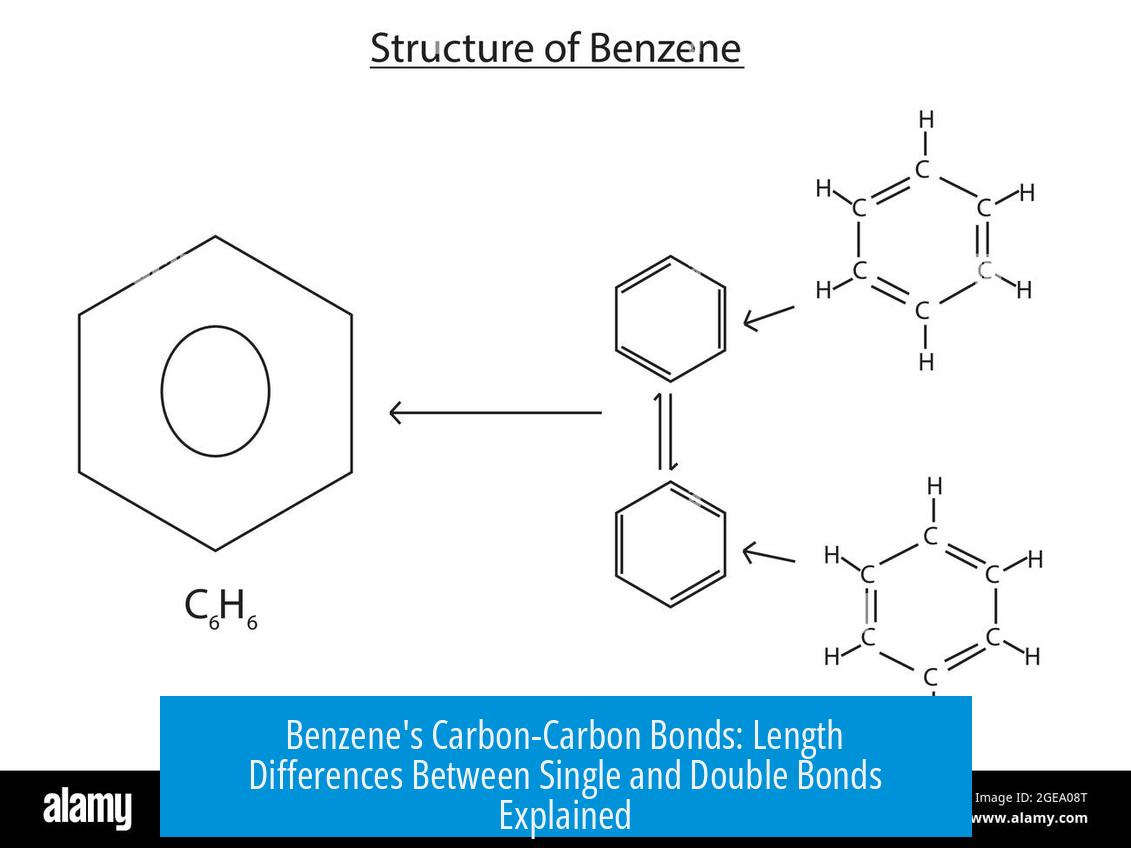
Benzene’s structure departs from simple single/double bond alternation. Its electrons are delocalized across the entire six-carbon ring, forming a system of conjugated pi electrons.
This delocalization equalizes bond character, making each bond have a bond order of about 1.5 instead of a clear single or double bond.
Consequently, all carbon-carbon bonds in benzene have lengths between those typical of single and double bonds. This manifests as uniform bond lengths and strengths across the ring.
Resonance Explanation
Resonance theory clarifies this phenomenon by describing benzene as a hybrid of multiple structures rather than alternating single and double bonds.
This hybridization leads to equivalence in all bonds and the observed intermediate bond length that resists localization into distinct single or double bonds.
“Kekulé’s alternating bond model is insufficient for benzene’s bonding; resonance provides the correct framework for understanding its bond lengths.”
Key Takeaways
- Bond length balances nucleus repulsion and electron-nucleus attraction forces.
- Double bonds are shorter than single bonds due to greater electron density.
- Benzene’s carbon-carbon bonds are intermediate in length due to electron delocalization.
- Resonance theory explains benzene’s uniform, intermediate bond lengths.
Benzene: How and Why Do Carbon-Carbon Single and Double Bonds Have Different Lengths? And How Can a Carbon-Carbon Bond Be In-Between?
Ever wondered why some bonds stretch longer while others seem to squeeze tighter? Specifically, why do carbon-carbon single and double bonds have different lengths? And how can some bonds, like those in benzene, be somewhere in the middle? Let’s dive in and unravel this chemical mystery step by step.
The Tug of War That Defines Bond Length
Picture a tiny tug-of-war happening inside molecules. On one side, you have the positively charged nuclei trying to keep their distance—they repel each other. On the other, negatively charged electrons act like matchmakers, pulling these nuclei together.
This delicate balance between repulsive and attractive forces decides the bond length—the exact gap where the forces cancel out and the bond settles in.
- The nuclei are both positively charged and don’t like being too close—it’s like two magnets flipping the same pole at each other.
- But the electrons, negatively charged, provide a glue, attracting both nuclei toward themselves.
- When these opposing forces hit equilibrium, the bond length is born.
Why Single Bonds Are Longer Than Double Bonds
Now hold onto your lab goggles—here’s the juicy part. A single carbon-carbon bond involves two electrons sharing the space and acting like a cozy bridge. But a double bond doubles the electron party, with four electrons hanging out between the atoms.
More electrons mean more attraction. Imagine the nuclei are like dance partners held together tighter by extra hands. The result? The partners draw closer, shortening the bond.
- Single bonds have 2 bonding electrons; double bonds have 4.
- More electrons increase the pull between nuclei, reducing the bond length.
- So, double bonds are shorter and stronger than single bonds.
To put numbers on it: a typical C-C single bond is about 1.54 Å (angstroms) long, whereas a C=C double bond shortens to roughly 1.34 Å. That difference might seem tiny, but it’s huge in the microscopic world!
How Benzene Bonds Are the Goldilocks of Bond Lengths
Now, benzene breaks the rules and laughs in the face of simple single/double bond conventions. For ages, chemists thought benzene had alternating single and double carbon bonds, like a “zigzag” fashion. But here’s the twist: benzene’s electrons don’t commit—they’re delocalized.
Delocalization means the electrons don’t stick between just two atoms. Instead, they spread out evenly over the entire hexagonal ring, creating a bond that’s not quite single and not quite double.
This gives each bond a “bond order” of about 1.5. What does that mean? It means each bond is halfway between a single and double bond, settling at an intermediate length—around 1.39 Å. Not too long, not too short; just right.
That’s why chemists love to draw benzene with a circle inside the ring instead of alternating double and single bonds. It’s a visual cue that electrons are partying all around the ring, not just chatting bipartite.
Resonance: The Ultimate Explainer
If you’re scratching your head wondering how this electron delocalization works, welcome to resonance theory—the rockstar of benzene chemistry.
Back in the day, August Kekulé imagined benzene with alternating double bonds. But experiments spoiled his party: all benzene bonds appeared identical in length and behavior.
Resonance theory swoops in to save the day, showing that benzene isn’t stuck in one static arrangement but rather a hybrid—an average—of multiple resonance structures. This hybridization spreads the electrons uniformly, stabilizes the molecule, and creates those equal, intermediate bond lengths.
What Does All This Mean in Real Life?
Understanding the bond lengths in benzene isn’t just academic trivia; it’s crucial for industries dealing with plastics, pharmaceuticals, and fuels. The unique stability of benzene’s ring comes from these special bonds, affecting reactivity and chemical behavior.
Chemists designing new molecules often exploit this balance. For example, they might tinker with substituents on the benzene ring to influence electron delocalization, tweaking properties like solubility or strength.
A quick tip for students or science lovers: When you come across molecules that look like they have alternating bonds, pause and ask, “Could resonance or delocalization be at play here?” It might save you from a super wrong interpretation.
Summary Table: Comparing Carbon-Carbon Bonds
| Bond Type | Number of Bonding Electrons | Approx. Bond Length (Å) | Bond Strength |
|---|---|---|---|
| Single (C-C) | 2 | 1.54 | Weaker, longer bond |
| Double (C=C) | 4 | 1.34 | Stronger, shorter bond |
| Benzene (delocalized) | ~3 (spread over ring) | 1.39 | Intermediate strength and length |
Final Thought: Chemistry’s Middle Ground
So, how and why do carbon-carbon single and double bonds have different lengths? Because the number of electrons in the bond zone dictates the push-pull forces between nuclei. More electrons pull nuclei closer, shortening the bond.
And benzene? It laughs at this binary bandwagon and introduces the concept of electron delocalization. Benzene’s carbon-carbon bonds split the difference, neither fully single nor fully double, but an elegant in-between that defines its chemical charm.
Next time you look at a molecular diagram of benzene, remember: those bonds are superheroes of the chemistry world—not just simple singles or doubles but a perfectly balanced dance of electrons, making benzene one of the most fascinating molecules around.
Why do carbon-carbon single and double bonds have different lengths?
Single bonds have 2 bonding electrons, while double bonds have 4. The extra electrons in double bonds increase attraction and pull nuclei closer, making double bonds shorter.
What balances the length of a carbon-carbon bond?
Bond length is where two forces balance: repulsion between the positively charged nuclei and attraction between each nucleus and the bonding electrons.
How can a carbon-carbon bond length be between that of a single and double bond in benzene?
In benzene, electrons are delocalised around the ring, not locked in single or double bonds. This creates bonds with a bond order of about 1.5, making bond lengths intermediate.
What does electron delocalisation mean in benzene?
Electrons move freely over the entire ring structure, spreading out the bonding effect evenly. This prevents bonds from being purely single or double.
How does resonance explain benzene’s bond lengths?
Resonance theory shows benzene’s bonds as a hybrid of multiple structures. Bonds appear identical and have lengths between single and double bonds, matching observations.




Leave a Comment