Understanding Mechanisms in Carbocation Rearrangement Examples
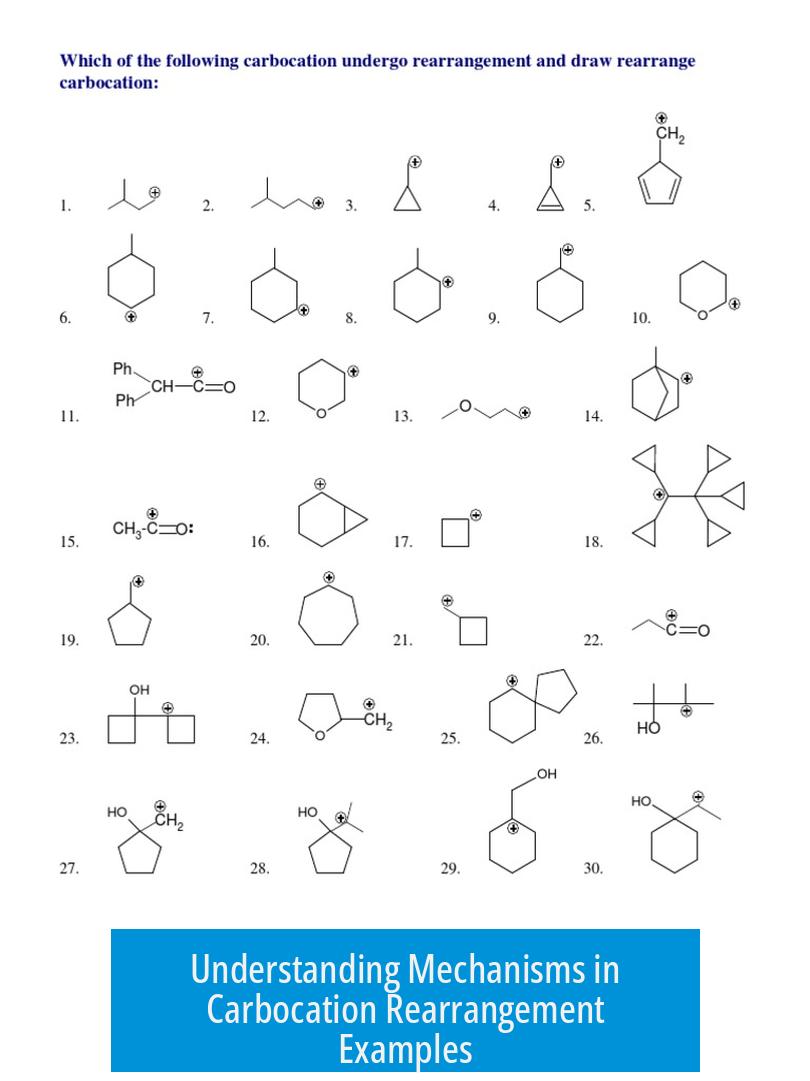
Carbocation rearrangement mechanisms involve shifts of hydrides or alkyl groups to form more stable carbocations, driven by the molecule’s attempt to increase stability. These shifts occur through well-defined electron movements, depicted by arrows that always point from electron-rich regions to electron-poor carbocation centers.
Why Arrows Never Originate from Carbocations
In reaction mechanism diagrams, arrows show the movement of electrons. Electrons come only from nucleophiles or electron-rich bonds. Carbocations, which are positively charged species, lack electrons, so they cannot donate electrons. Therefore, arrows never come from a carbocation’s positive charge.
This means the carbocation acts as an electrophile—an electron acceptor. Arrows must always point towards the carbocation, showing electrons flowing into the positively charged center from a neighboring bond or lone pair. This is the fundamental rule in arrow pushing for carbocations.
Correct Direction of Arrows in Carbocation Mechanisms
Confusion arises when one thinks of carbocations as “attacking” something else. This is incorrect. Carbocations are electron-deficient; they do not attack but rather are attacked by electron-rich species.
- Always draw arrows showing electron motion from the nucleophile or bond to the carbocation.
- Never depict electrons moving away from the carbocation positive charge.
- This reinforces the concept that electrophiles accept electrons, nucleophiles donate electrons.
Structural Clarity: Numbering and Drawing Full Structures
During carbocation rearrangements, atoms move and charges shift. Tracking these changes requires clear structural drawings. Numbering carbons and showing all hydrogens clarifies what bonds break and form.
This practice is especially important in ring expansions or complicated rearrangements. Full structures prevent errors such as losing track of which carbon carries the positive charge or confusing which hydride or alkyl group migrates.
Common Mistakes in Carbocation Rearrangement Mechanisms
Some typical errors include incorrect bond formations or generating unrealistic intermediates with multiple positive charges, which are unstable.
- A double bond cannot form a bond directly to a secondary carbon if it results in multiple positive charges or an unrealistic pentavalent carbon structure.
- Attempting such moves violates basic valence rules and stability principles.
Recognizing these mistakes helps avoid proposing mechanistically impossible pathways.
Resonance and Carbocation Stability Guide Reaction Direction
Carbocations stabilize through resonance or shifts that create more substituted carbocation centers.
Consider two possible rearranged structures:
- One that gains resonance stabilization by delocalizing the positive charge.
- Another forming a secondary but less resonance-stabilized carbocation.
The resonance-stabilized carbocation typically predominates, making the rearrangement toward that structure more favorable.
Fundamentals to Master: Hydride and Alkyl Shifts with Proper Arrow Pushing
Before delving into complex carbocation rearrangements, mastering the basics is essential. This includes understanding hydride shifts and alkyl shifts.
- Hydride shifts involve a hydrogen atom with its bonding electrons moving from an adjacent carbon to the carbocation center.
- Alkyl shifts are similar but involve alkyl groups migrating with their bonding electrons.
- In every step, arrows must show electron pair shifts toward the carbocation, not away.
Video tutorials and well-organized practice can reinforce these principles. Understanding electron flow is the foundation for all rearrangement mechanism mastery.
Detailed Mechanism Examples of Carbocation Rearrangements
Example 1: Hydride Shift
Consider a carbocation at a secondary carbon adjacent to a tertiary carbon.
- The tertiary carbon has a C–H bond next to the carbocation.
- Arrow pushing shows the pair of electrons in that C–H bond moving toward the carbocation carbon.
- The hydride (H−) moves, shifting the positive charge to the tertiary carbon where it is more stable due to greater alkyl substitution.
This migration increases carbocation stability. The arrow origin is from the electron-rich bond (C–H) to the deficient carbocation center.
Example 2: Alkyl Shift with Ring Expansion
In ring systems, carbocation migration might cause ring expansion. Numbering carbons helps clarify this complex process.
- Identify the carbocation carbon and adjacent carbon bearing the alkyl group.
- Draw an arrow from the C–C bond between these carbons towards the carbocation center.
- The alkyl group moves, and the former carbocation carbon becomes neutral.
- The positive charge relocates to the carbon where the alkyl group was originally bonded.
- This may result in a larger ring, stabilizing the carbocation by relieving ring strain.
Full structural drawings prevent mix-ups in the migrating group’s identity and help visualize the charge shift.
Common Misinterpretations in Examples
Some attempts incorrectly show double bonds attacking carbons, resulting in multiply charged intermediates or violating octet rules.
In the correct mechanisms:
- Carbocations accept electrons but do not provide electrons to form new bonds.
- Multiplying positive charges on carbons or pentavalent carbons signal structural errors.
- Ensure all carbons obey valence rules and charge distribution is chemically reasonable.
Summary of Best Practices in Carbocation Rearrangement Mechanisms
- Always draw arrows from electron-rich bonds or lone pairs to carbocation centers.
- Never draw arrows starting from the positive charge of carbocations.
- Number carbons and show all atoms to keep track of rearrangements clearly.
- Recognize and avoid impossible bond formations and highly unstable intermediates.
- Apply resonance concepts to predict favored carbocation rearrangements.
- Master hydride and alkyl shifts with accurate electron flow before moving to complex examples.
Why should arrows in carbocation mechanisms never start from the positive charge?
Arrows show electron movement. Carbocations lack electrons, so arrows must start from electron-rich areas toward the carbocation. Drawing arrows from the positive charge is incorrect because it shows electrons coming from a place with none.
How can numbering carbons and drawing full structures help in carbocation rearrangements?
Numbering carbons and drawing all atoms clearly helps track where electrons and charges move. It avoids confusion, especially in complex shifts or ring expansions, by making each step easy to follow.
Why is it wrong to show a double bond forming a bond with a secondary carbon during rearrangement?
Such a step creates unrealistic species with multiple positive charges and unstable structures. Mechanisms must avoid forming impossible intermediates to remain chemically plausible.
How does resonance affect carbocation rearrangement pathways?
Resonance stabilizes carbocations, making some rearrangements more favorable. Carbocations that gain resonance or form more stable centers, like secondary carbocations, tend to be preferred.
What fundamental concepts should I focus on to understand carbocation rearrangements better?
- Learn correct arrow pushing: always from electron-rich sites to carbocations.
- Understand hydride and alkyl shifts as common rearrangement types.
- Practice drawing clear and full structures to track changes.





Leave a Comment