How Are These Enantiomers?

These molecules qualify as enantiomers because they are non-superimposable mirror images of each other.
This precise relationship arises when two stereoisomers have chiral centers, and one is the exact mirror image of the other, yet cannot be perfectly overlaid by rotation or translation. The molecules are thus mirror image stereoisomers.
Definition and Core Concept
Enantiomers are pairs of molecules that are mirror images but not superimposable. Unlike meso compounds, which have chiral centers but possess an internal plane of symmetry, enantiomers lack any such symmetry. Each molecule in the pair has chiral centers but no plane within the molecule allows symmetry, confirming they are not meso.
- Non-superimposable mirror images.
- Mirror image stereoisomers with chiral centers present.
- No internal plane of symmetry; hence, not meso.
Visual Verification Through Rotation and Flipping
One way to confirm enantiomerism involves physically manipulating the molecular structures:
- Rotate both molecules 90° counterclockwise (CCW) to visualize a clear mirror plane.
- Hold the molecule by one bromide and spin it to compare spatial orientation.
- Flip one molecule across the x-axis.
Flipping on the x-axis causes several changes:
- Bromines switch from top to bottom positions.
- Wedge bonds become dash bonds; dash bonds become wedges, indicating changes in 3D orientation.
- Hydroxy groups swap between projecting into and out of the plane.
Asterisks can be placed on bromines and hydroxy groups to track these shifts. Importantly, after flipping, the absolute configuration at each chiral center remains the same in spatial terms, verifying a “correct” flipping (like flipping a pancake). However, because wedges became dashes and vice versa, all chiral centers’ R/S configurations invert. This inversion of every chiral center confirms the molecules as enantiomers.
Absolute Configuration and CIP Rules
Assigning Cahn-Ingold-Prelog (CIP) priority rules provides a rigorous method to identify enantiomers:
- Calculate R or S designations at each chiral center before flipping.
- After flipping, check if all chiral centers invert their configurations (R → S and S → R).
If all centers invert, the molecules are enantiomers. Partial inversion would indicate diastereomers. For the molecules in question, all stereocenters change configuration upon flipping, a definitive mark of enantiomerism.
Superimposability and Mirror Image Relations
Attempting to superimpose the two molecules fails due to differences in hydroxyl group positions. However, aligning the oxygen atoms within the ring structure shows the molecules as mirrors.
Ignoring stereochemistry, the structures appear identical. Yet, the spatial arrangement of wedges and dashes demonstrates that the molecules differ in three-dimensional orientation. Rotating one molecule 180° along a horizontal axis and then reflecting it across a plane produces the other, consistent with mirror images that are non-superimposable.
| Operation | Effect |
|---|---|
| Rotate 180° horizontally | Bromines change direction (going into the plane), oxygen positions visually stay consistent |
| Reflect in the plane of the page | Creates mirrored structure, confirming mirror-image relationship |
Common Confusions and Clarifications
Some may mistake these molecules as diastereomers through mental models that do not incorporate flipping wedges and dashes or omit analyzing all chiral centers. Key points clarify such misinterpretations:
- Wedges and dashes do flip when rotating and mirroring, affecting designation.
- All chiral centers invert their absolute configuration; a distinctive feature of enantiomers.
- Maintaining wedge orientation on bromines does not imply identical compounds because the rest of the stereochemistry changes.
It is the uniform inversion of stereochemistry that distinguishes enantiomers from diastereomers, which differ at some but not all chiral centers.
Practical Verification Through Model Building
Constructing physical models facilitates visualizing the inversion of stereocenters:
- Build one stereoisomer model and then flip it over to mimic mirroring.
- Observe how all stereocenters’ configurations invert after flipping.
- Confirm the molecules cannot be superimposed but are mirror images.
This hands-on approach resolves ambiguous mental attempts and demonstrates unequivocally that the molecules are enantiomers.
Summary of Key Concepts
- Enantiomers are non-superimposable mirror image stereoisomers.
- They contain chiral centers but lack internal symmetry planes, differentiating them from meso compounds.
- Flipping or reflecting a molecule inverts all its chiral centers’ configurations (R to S and vice versa).
- ROtating and flipping molecules while tracking wedges and dashes helps visualize enantiomerism.
- Assigning absolute configuration through CIP rules confirms complete inversion of stereochemistry.
- Physical model building aids clear confirmation of enantiomeric relationships.
How Are These Enantiomers? Unpacking the Mystery of Mirror-Image Molecules
Enantiomers are non-superimposable mirror images. That’s the fundamental answer. Imagine holding two gloves: your left and right hands. They look alike but can’t perfectly sit on top of each other. That’s how enantiomers behave in the molecular world—like mirror twins who just can’t “wear” the same shape.
This simple but powerful idea holds the key to understanding stereochemistry puzzles. Enantiomers are mirror image stereoisomers, meaning they share the same molecular formula yet differ in the 3D orientation of atoms around chiral centers.
Chiral Centers and Why These Are Not Meso
One might wonder, “Are they meso compounds? Because meso molecules are symmetric and achiral despite having chiral centers.” Here, the answer is no. These molecules possess chiral centers but lack an internal plane of symmetry. The absence of that plane means they do not cancel the chirality internally and are thus not meso compounds.
You can safely say, “They are definitely chiral molecules and not meso because the internal symmetry—the magic eraser of chirality—is missing.”
How Can We Visually Confirm Enantiomerism?
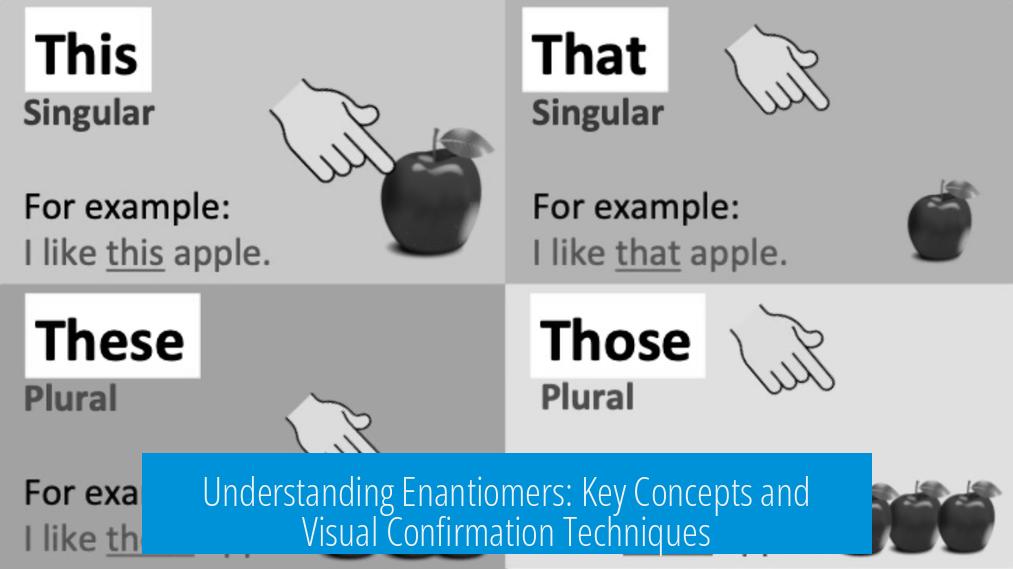
Envision this visualization challenge: Take both molecular structures and rotate them 90 degrees counterclockwise (CCW). Suddenly, the mirror plane becomes obvious. You clearly see how one structure reflects off this plane to become the other. Mind-blowing, right?
Want to get hands-on? Grab one bromide (a bromine atom bonded in the molecule) and spin it like a tiny globe. This physical “fidget” helps make the mirror image relationship palpable.
Try flipping the left molecule across the x-axis. Watch what happens:
- Bromines swap positions from top to bottom.
- Wedge bonds toggle to dash bonds, while dash bonds flip to wedges.
- Hydroxy (-OH) groups alter their spatial orientation, flipping inside and out of the plane.
Adding asterisks on bromines and hydroxy groups helps track these flips—like leaving breadcrumbs in a forest of atoms.
This flipping is like turning a pancake over—it doesn’t create a new pancake but just reverses what you see. To double-check, assess the absolute configurations (R or S) of each chiral center before and after flipping. If all configurations remain consistent on the flipped molecule, it confirms you flipped correctly.
Yet, here’s the kicker: while flipping changes wedges to dashes and vice versa, all R configurations turn into S, and all S turn into R. That inversion in all chiral centers’ absolute configurations is the smoking gun proving these molecules are enantiomers, not just similar siblings.
For peace of mind, build a model—yes, in real life with molecular kits—and physically flip the molecule. The inversion becomes crystal clear. Models do not lie; they confirm these are 100% enantiomers.
Why Can’t We Superimpose These Molecules?
Superimposability is a tricky concept. It’s tempting to think, “Hey, I can just overlay them!” But no. The positions of hydroxyl groups block perfect superimposition. Their spatial orientations are different enough that even if you rotate one molecule to align the ring oxygen with the other, the hydroxyl positions still prevent a perfect overlay.
Ignoring stereochemistry altogether—that is, ignoring wedges and dashes—would make the molecules look identical. Without stereochemical detail, nothing separates them. The twist lies in the 3D spatial arrangements, subtle yet crucial.
An effective way to relate these is:
- Rotate the left molecule 180 degrees along the horizontal axis.
- The bromines now point away, but the oxygens keep their relative orientation.
- Mirror this setup across the plane of the page to get the molecule on the right.
This sequence confirms a mirror image relationship, explaining why these molecules qualify as enantiomers.
The Role of the Cahn-Ingold-Prelog (CIP) Rules
Ever heard of the CIP priority system? It’s the gold standard in stereochemistry for assigning R or S configuration to chiral centers. Using this system, you assess atomic priorities around each chiral center and assign R or S labels accordingly.
Here’s the beauty: if every single chiral center flips its configuration from R to S or vice versa, your molecules are enantiomers. Partial flipping means they are diastereomers, but complete flipping is an enantiomer hallmark.
Applying this rule can be time-consuming, but it provides precise proof of enantiomerism. For instance, after flipping the molecule, every wedge turns into a dash and every dash into a wedge, signaling that all stereocenters invert configuration.
Common Confusions and How to Navigate Them
Understanding enantiomers isn’t always straightforward. Mental mirroring can get messy, sometimes making molecules look like diastereomers instead. Such confusion often arises because:
- Sometimes wedges and dashes don’t seem flipped as you expect.
- There are constitutional planes of symmetry to consider that complicate “mirror flipping.”
- Questions arise if the R and S assignments of bromines stay the same.
Here’s a handy tip: for the molecule to be identical and not just an enantiomer, every wedge/dash needs to flip correspondingly. If any bromine remains wedged while the rest invert, it signals a difference—in fact, a clear marker that you’re not dealing with the same molecule.
Imagine flipping the molecule like a pancake. If bromines stay on the right side and wedges/dashes flip, you’re looking straight at the mirror image. This perspective helps untangle the confusion.
Tools and Recommendations to Confirm Enantiomeric Relationship
Books and drawings only get you so far. Building actual molecular models adds clarity. Grab a model kit, construct both molecules, and flip one over. Seeing the stereocenters invert physically helps cement your understanding.
Also, visualize an axial plane as a mirror in space. Picture the molecule “reflected” in this plane—it changes conformation exactly as a mirror image should if it’s an enantiomer. This mental exercise helps solidify spatial reasoning.
Why Does This Matter? The Significance of Recognizing Enantiomers
So why should you care if molecules are enantiomers? Because enantiomers can behave radically differently in biology and chemistry. One may be a lifesaver drug; the other, a harmful or inert substance. Their mirror relationship affects enzyme binding, receptor interaction, and chemical reactivity.
Accurate identification aids organic synthesis, pharmaceutical design, and academic understanding. Molecular chirality is at the heart of many biochemical processes.
Summary: Enantiomers as Non-Superimposable Mirror Images
In essence, these enantiomers differ because they are non-superimposable mirror images. Confirmed through rotation, flipping, and CIP configuration assignments, every chiral center flips from R to S or S to R. Visual clues—the inversion of wedges and dashes, positional changes in bromines and hydroxy groups—strengthen this conclusion. Using molecular models and thoughtful visualization clears the mental fog.
So next time you face a pair of puzzling molecules, remember: rotate like a chemist, flip like a pancake, and always check those configurations. You’ll spot enantiomers with confidence—and maybe impress your chemistry pals with your stereochemical savvy!


Leave a Comment