Understanding Planar Structures and Aromaticity
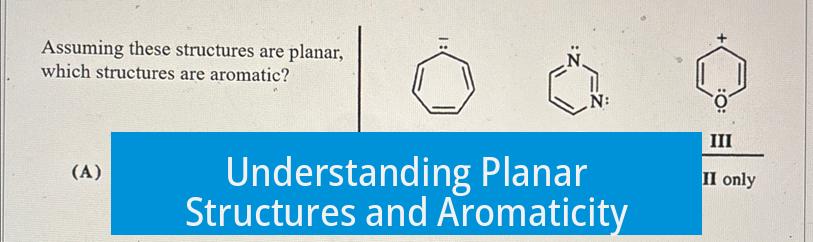
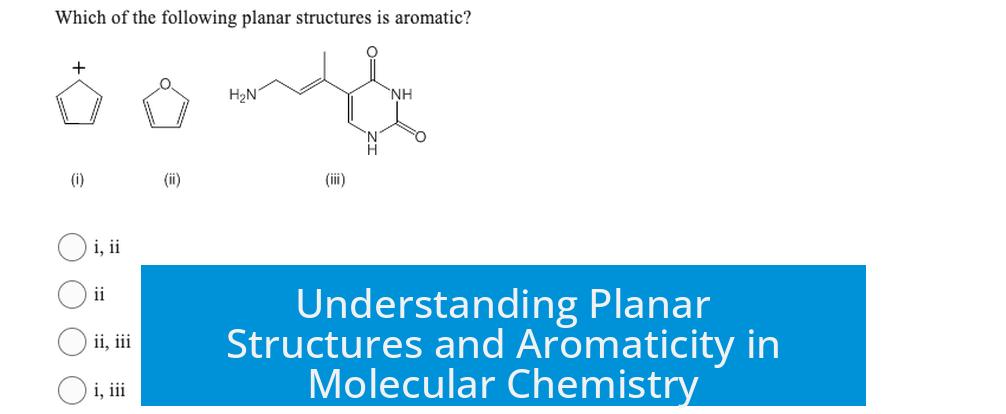
Aromaticity describes a continuum of stabilization, not a strict yes-or-no property. Molecules can exhibit varying degrees of aromatic character depending on their structural features.
Aromaticity as a Scale
Aromaticity is better seen as a spectrum. Systems range from strongly aromatic to weakly aromatic. This scale reflects how well the molecule stabilizes through electron delocalization in a cyclic, conjugated π-system.
Planarity’s Role in Aromaticity
Planarity allows p-orbitals to overlap effectively and enable delocalized electron flow. When a cyclic compound meets the 4n + 2 π-electron rule, there is a natural tendency for it to become planar to maximize aromatic stabilization.
However, achieving perfect planarity is not always possible. Molecular strain or steric factors can distort the ring, reducing or eliminating aromatic character. Thus, even if the molecule satisfies electron count rules, it may only be weakly aromatic or non-aromatic if planarity is compromised.
Impact of Ring Size and Bond Angles
- Rings with seven or more atoms usually cannot maintain planarity due to geometric strain.
- Bond angles are critical. For example, some rings require bond angles around 144°, incompatible with planar structures which favor approximately 120° angles for effective overlap.
- Non-planar rings cannot conduct effective π-electron delocalization, losing aromatic stabilization entirely.
Beyond Simple Rules: Real Molecular Complexity
The 4n + 2 rule and the assumption of planarity are excellent starting points for understanding aromaticity. However, real molecules often behave with more complexity. Visualization tools or molecular models help clarify whether a system truly attains aromatic planarity.
Building molecular models is a practical method to see how ring size and geometry influence planarity. It reinforces why some theoretically aromatic rings distort to non-planar conformations.
Key Points to Remember
- Aromaticity varies in strength; it is not strictly present or absent.
- Planarity facilitates electron delocalization, essential for aromatic stabilization.
- The 4n + 2 rule guides aromaticity predictions but does not guarantee planarity.
- Large rings and improper bond angles often prevent a molecule from being planar and aromatic.
- Examining models or computational data helps reveal real-world aromatic behavior.
What does it mean that aromaticity is a scale and not binary?
Aromaticity can vary in strength. Some molecules are strongly aromatic while others show weak aromatic behavior, depending on how well they meet certain conditions.
How does planarity affect aromaticity in cyclic molecules?
Planarity allows for better overlap of p-orbitals, providing aromatic stabilization if the molecule follows the 4n+2 rule. If a ring is not planar, it may lose aromaticity or become weakly aromatic.
Why do larger rings tend to be non-planar and non-aromatic?
Rings with seven or more carbons often cannot maintain a planar shape due to strain, which disrupts the continuous overlap of orbitals needed for aromaticity.
Can bond angles prevent a molecule from being aromatic?
Yes. If the bond angles required for planarity are too different from ideal angles, the molecule may distort and lose planarity, preventing it from being aromatic.
Is it always possible to tell if a molecule is aromatic just by looking at its structure?
No. Aromaticity involves subtle structural features and strain effects. Models or calculations may be needed to confirm if a ring is planar and aromatic despite following simple rules.


Leave a Comment