Differences Between SN1 and SN2 Reactions
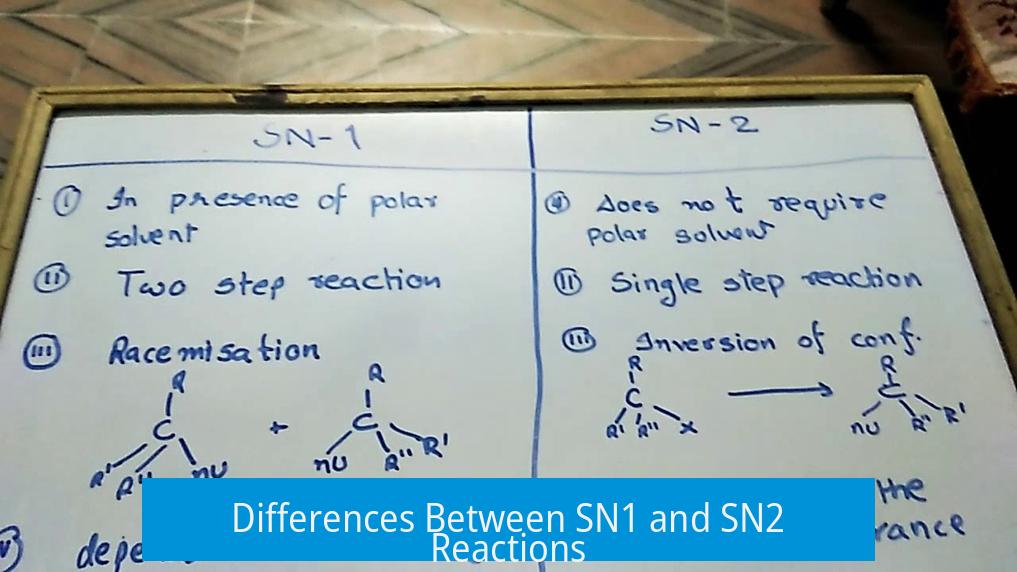
SN1 and SN2 reactions differ mainly in their reaction mechanisms, rate-determining steps, stereochemistry, substrate type, and reaction conditions. Understanding these distinctions clarifies how nucleophilic substitution proceeds under various chemical environments.
1. Rate-Determining Step and Kinetics
SN1 reactions follow a unimolecular rate-determining step. This means the slowest step depends on the concentration of only one species: the substrate.
- In the first step, the leaving group departs, forming a carbocation.
- The carbocation formation controls the overall reaction rate.
In contrast, SN2 reactions exhibit a bimolecular rate-determining step. Two species affect the rate:
- The nucleophile attacks the substrate at the same time the leaving group leaves.
- This concerted one-step mechanism directly relates rate to nucleophile and substrate concentrations.
2. Reaction Mechanism and Carbocation Formation

SN1 proceeds via two steps:
- Leaving group dissociates, forming a planar carbocation intermediate.
- Nucleophile attacks the carbocation.
The carbocation intermediate is critical; it influences the reaction’s stereochemistry and rate.
SN2, on the other hand, avoids carbocation formation:
- The nucleophile performs a backside attack in a single concerted step.
- No intermediate forms; breaking and making bonds occur simultaneously.
3. Stereochemistry Outcomes
Stereochemistry distinguishes SN1 and SN2 markedly:
| Reaction Type | Stereochemical Result |
|---|---|
| SN1 | Racemic mixture formation due to nucleophilic attack from either side of planar carbocation |
| SN2 | Stereoinversion from backside attack, often called Walden inversion |
The carbocation intermediate in SN1 has an empty p orbital accessible to nucleophiles from both sides, leading to loss of stereochemical purity.
4. Substrate and Carbocation Stability
Substrate structure heavily influences whether SN1 or SN2 dominates:
- SN1 favors substrates that form stable carbocations, usually tertiary > secondary > primary (with primary rarely reacting via SN1).
- SN2 favors less hindered, primary substrates for an unhindered backside attack, sometimes secondary; tertiary substrates are sterically hindered and rarely undergo SN2.
5. Solvent Effects
Solvent choice affects the reaction pathway:
- SN1 is favored by protic solvents like water or alcohols. Protic solvents stabilize the carbocation and the leaving group by hydrogen bonding.
- SN2 is favored by aprotic solvents such as DMSO or acetone, which do not form strong hydrogen bonds. This leaves nucleophiles more reactive for backside attack.
6. Leaving Group Requirements
Both SN1 and SN2 require good leaving groups. The quality of the leaving group affects reaction rate and success. Poor leaving groups impede both mechanisms.
7. Analogies Clarifying Mechanistic Differences
- SN1: The leaving group “gets up from its chair,” creating an empty seat (carbocation). Later, the nucleophile “sits down” from either side.
- SN2: The nucleophile “pushes” the leaving group out in one concerted effort, knocking the incumbent out of its seat directly.
8. Summary Table of SN1 vs SN2
| Aspect | SN1 | SN2 |
|---|---|---|
| Rate Law | Unimolecular (rate depends on substrate) | Bimolecular (rate depends on substrate and nucleophile) |
| Mechanism | Two steps: carbocation intermediate | One step: concerted attack and leaving |
| Carbocation | Formed | Not formed |
| Stereochemistry | Racemic mixture | Stereoinversion |
| Substrate | Favors tertiary, stable carbocations | Favors primary, less hindered |
| Solvent | Protic solvents | Aprotic solvents |
| Leaving Group | Good leaving group needed | Good leaving group needed |
9. Additional Considerations
Carbocation rearrangements occur during SN1, complicating product distributions. SN2 involves no rearrangements due to its one-step nature.
The nucleophile in SN1 reactions may be weak, as it attacks a positively charged carbocation. SN2 requires a strong nucleophile to simultaneously attack and displace the leaving group.
Experimental tools like kinetic studies help determine the pathway by measuring rate dependence on nucleophile concentration.
Key Takeaways
- SN1 proceeds via carbocation intermediate with a unimolecular rate-determining step and yields racemic mixtures.
- SN2 occurs in one step with simultaneous nucleophile attack and leaving group departure, causing stereoinversion.
- Substrate structure and solvent choice strongly influence the mechanism.
- Protic solvents favor SN1; aprotic solvents favor SN2.
- Stable carbocations favor SN1; hindered substrates favor SN2.
- Both mechanisms require a good leaving group.
For visualization and practice, instructional videos offer detailed example problems and comparisons on kinetics, nucleophile effects, substrates, solvents, and stereochemical outcomes. These help deepen understanding beyond this concise overview.





Leave a Comment