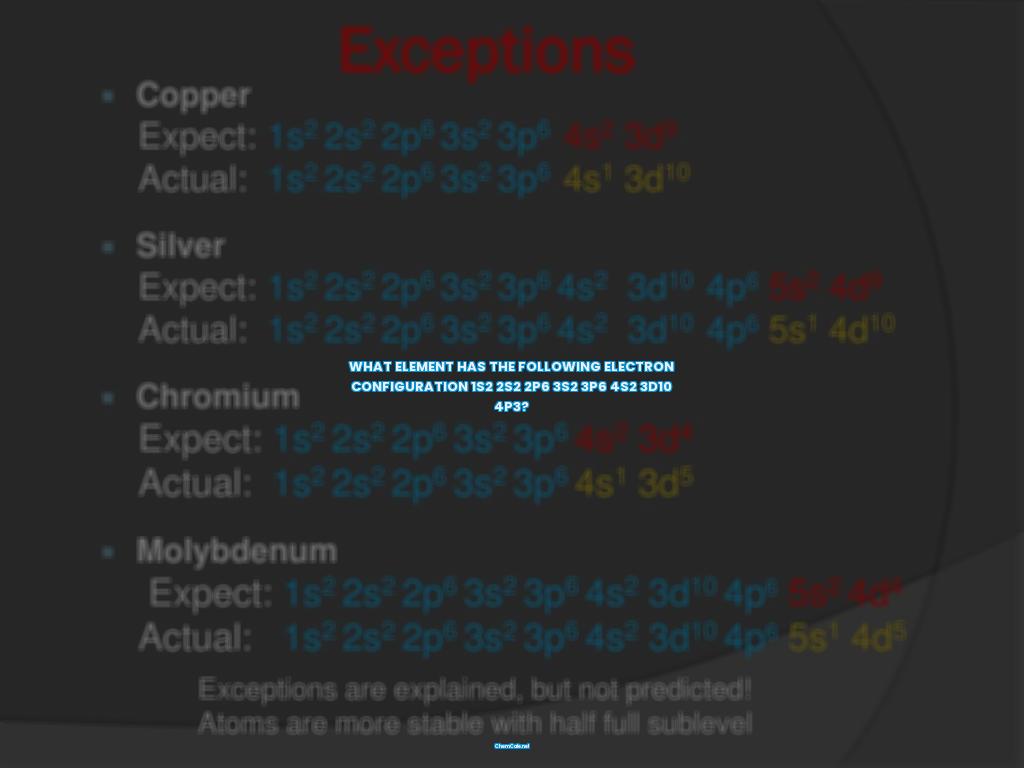
Have you ever wondered which element has the electron configuration 1s2 2s2 2p6 3s2 3p6 4s2 3d10 4p3? Well, it’s time to find out! This is a common question that comes up in chemistry, and the answer might surprise you.
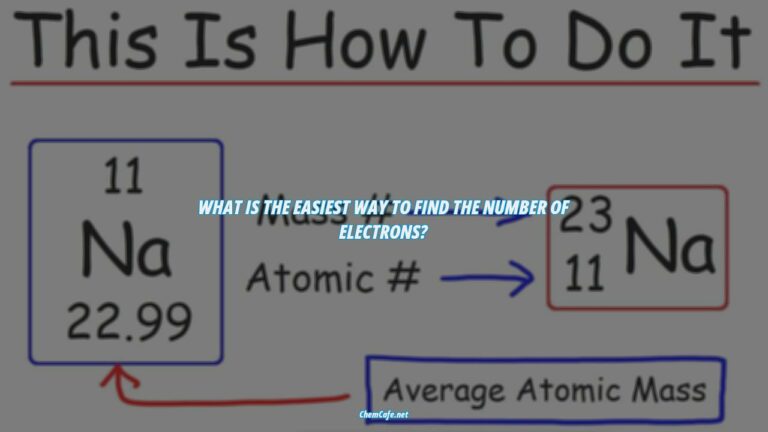
The electron configuration of an element is a representation of the arrangement of electrons around its nucleus. It is the basis of understanding the periodic table of elements and the various properties of each element. The electron configuration of an element is determined by the number of protons and neutrons in the nucleus, as well as the number of electrons in each energy level.
The electron configuration 1s2 2s2 2p6 3s2 3p6 4s2 3d10 4p3 indicates that there are fourteen electrons in total. This is because the superscripts (2, 2, 6, 2, and 3) are the number of electrons in each orbital. Just by adding up these numbers, we can determine that there are fourteen electrons in total.
Now comes the next step: finding the element that has this electron configuration. To do this, we simply look for the element on the periodic table with the atomic number of fourteen. It turns out that the elements osmium (Os) and iridium (Ir) both have this electron configuration. Osmium has an atomic weight of 190.2, and its group is Transition Elements. Iridium has an atomic weight of 192.2, and its group is also Transition Elements.
So there you have it! The electron configuration 1s2 2s2 2p6 3s2 3p6 4s2 3d10 4p3 indicates that there are fourteen electrons in total and that the elements osmium and iridium both have this electron configuration. Are you surprised? Now you know the answer to the question, “What element has the following electron configuration?”
What element has the following electron configuration 1s2 2s2 2p6 3s2 3p6 4s2 3d10 4p3?
Have you ever wondered what element has the electron configuration 1s2 2s2 2p6 3s2 3p6 4s2 3d10 4p3? It can be a bit confusing at first, but with a little help, you can easily figure it out.
The first step is to identify the electron configuration of the element in question. In this case, the electron configuration is 1s2 2s2 2p6 3s2 3p6 4s2 3d10 4p3. The supercripts (2, 2, 6, 2 and 2) are the number of electrons in each orbital. Simply add up these numbers, which will give you 14. Now just look for the element on the periodic table with the atomic number of 14.
The element is Osmium. It has an atomic weight of 190.2, an atomic number of 76, and is classified as a transition element. Its full electron configuration is 1s2 2s2 2p6 3s2 3p6 4s2 3d10 4p6 5s2 4d10 5p6 6s2 4f14 5d6.
Osmium is a hard and brittle metal that is found in trace amounts in nature. It is used in a variety of applications, including electrical contacts, electrodes, and spark plugs. It is also used in the production of alloys, such as osmiridium, and as a catalyst.
Another element with the electron configuration 1s2 2s2 2p6 3s2 3p6 4s2 3d10 4p3 is Iridium. It has an atomic weight of 192.2, an atomic number of 77, and is also classified as a transition element. Its full electron configuration is 1s2 2s2 2p6 3s2 3p6 4s2 3d10 4p6 5s2 4d10 5p6 6s2 4f14 5d7.
Iridium is a very hard and brittle metal that is found in trace amounts in nature. It is used in a variety of applications, including electrical contacts, electrodes, and spark plugs. It is also used in the production of alloys, such as iridosmium, and as a catalyst.
So there you have it! If you ever find yourself wondering what element has the electron configuration 1s2 2s2 2p6 3s2 3p6 4s2 3d10 4p3, you now know the answer: Osmium and Iridium. Both are transition elements, have atomic numbers of 76 and 77 respectively, and have full electron configurations of 1s2 2s2 2p6 3s2 3p6 4s2 3d10 4p6 5s2 4d10 5p6 6s2 4f14 5d6 and 1s2 2s2 2p6 3s2 3p6 4s2 3d10 4p6 5s2 4d10 5p6 6s2 4f14 5d7 respectively.
Which element has the following electron configuration 1s2 2s2 2p6 3s2 3p6 4s2 3d10 quizlet?
The electron configuration of an element is the arrangement of electrons in an atom or molecule. It determines the properties of the element and is an important concept in chemistry. If you know the electron configuration of an element, you can determine which element it is and its chemical properties.
If you come across an electron configuration such as 1s2 2s2 2p6 3s2 3p6 4s2 3d10, you can determine which element it is by looking at the atomic number of the element. The supercripts (2, 2, 6, 2 and 2) are the number of electrons in each orbital. Simply add up these numbers, which will give you 14. Now just look for the element on the periodic table with the atomic number of 14.
Sb – Antimony
Antimony is a chemical element with the symbol Sb and atomic number 51. It is a metalloid with a silvery white color and belongs to the group of non-metals. It has an electron configuration of 1s2 2s2 2p6 3s2 3p6 4s2 3d10 4p6 5s2 4d10 5p3. It has a melting point of 630.63 °C and a boiling point of 1635 °C. It is mainly used to make alloys and semiconductors.
Te – Tellurium
Tellurium is a chemical element with the symbol Te and atomic number 52. It is a metalloid with a silvery white color and belongs to the group of transition elements. It has an electron configuration of 1s2 2s2 2p6 3s2 3p6 4s2 3d10 4p6 5s2 4d10 5p4. It has a melting point of 449.51 °C and a boiling point of 990 °C. It is mainly used in electronics, alloys, and as an agricultural additive.
Ir – Iridium
Iridium is a chemical element with the symbol Ir and atomic number 77. It is a transition element with a silvery white color and belongs to the group of transition elements. It has an electron configuration of 1s2 2s2 2p6 3s2 3p6 4s2 3d10 4p6 5s2 4d10 5p6 6s2 4f14 5d7. It has a melting point of 2410 °C and a boiling point of 4527 °C. It is mainly used in electronics, alloys, and as a catalyst in chemical reactions.
So, the element with the electron configuration 1s2 2s2 2p6 3s2 3p6 4s2 3d10 is iridium. It is the element with atomic number 77 and belongs to the group of transition elements. Knowing the electron configuration of an element can help you determine its properties and its uses.
Which element has the following electron configuration 1s2 2s2 2p6 3s2 3p6 4s1?
The electron configuration of an atom is the arrangement of electrons in its atomic orbitals. It is a useful tool for predicting the properties of an element. The electron configuration of an element is typically written in a standard notation where all the electron-containing atomic subshells (with the number of electrons they hold written in superscript) are listed in a sequence.
For example, the electron configuration of sodium is 1s22s22p63s1. This notation can be lengthy for elements with a relatively large atomic number. But it is important to understand the concept of electron configurations in order to predict the properties of an element.
What is the Aufbau Principle?
The Aufbau principle states that electrons occupy the lowest energy subshell first. This principle is used to determine the electron configuration of an element. According to this principle, the energy level is represented by {eq}rm n {/eq} while the subshell is represented by {eq}rm l {/eq}. The number of electrons in the subshell is represented by {eq}rm e {/eq}.
What is the electron configuration of 1s2 2s2 2p6 3s2 3p6 4s1?
The electron configuration of 1s2 2s2 2p6 3s2 3p6 4s1 is the electron configuration of the element carbon. Carbon has an atomic number of 6, meaning it has 6 protons and 6 electrons. Its electron configuration can be written as 1s2 2s2 2p2. This means that the first energy level (1) is filled with two electrons, the second energy level (2) is filled with two electrons, and the third energy level (3) is filled with two electrons in the 2p subshell. This is the electron configuration of carbon.
What are the properties of elements with this electron configuration?
Elements with this electron configuration tend to have similar properties. For example, they tend to have a valence of 4, meaning that they tend to form four bonds when they interact with other elements. They also tend to have a high melting point and boiling point, making them solid at room temperature. Additionally, they tend to be non-metals and have a low electronegativity.
Electron configuration is an important concept in understanding the properties of elements. The electron configuration of an element is typically written in a standard notation, using the Aufbau principle. The electron configuration 1s2 2s2 2p6 3s2 3p6 4s1 is the electron configuration of the element carbon, and elements with this electron configuration tend to have similar properties.
What atom has this electron configuration 1s2 2s2 2p6 3s2 3p4?
Electron configuration is an important concept in chemistry that describes the arrangement of electrons in an atom. It can be used to identify elements, determine the chemical properties of elements, and predict the behavior of atoms. The electron configuration of an atom is usually written using a standard notation, which consists of the atomic number and then all electron-containing atomic subshells (with the number of electrons they hold written in superscript).
For example, the electron configuration of sodium is 1s22s22p63s1. But this notation can become quite lengthy for elements with a relatively large atomic number. So what atom does the electron configuration 1s2 2s2 2p6 3s2 3p4 correspond to?
Understanding Electron Configurations
To answer this question, it’s important to first understand what electron configurations are and how they are written. Electron configuration is a way of representing the electrons present in an atom. These electrons are distributed into orbital shells and subshells. The four different types of orbitals are the s, p, d, and f orbitals.
Each orbital shell can hold a maximum of two electrons. The outermost shell, or valence shell, is the most important shell because it determines the chemical properties of an atom. Electron configurations are written using a standard notation, which lists all the electrons in order of increasing energy.
The Electron Configuration of Sulfur
Now that we understand what electron configurations are, let’s look at the electron configuration 1s2 2s2 2p6 3s2 3p4. This electron configuration corresponds to the element sulfur.
Sulfur has an atomic number of 16, so it has 16 electrons. To determine the electron configuration of sulfur, we’ll start by putting two electrons in the 1s orbital. Then we’ll put two more electrons in the 2s orbital. We’ll put six in the 2p orbital and then put the next two electrons in the 3s. Since the 3s if now full we’ll move to the 3p where we’ll place the remaining four electrons. Therefore the sulfur electron configuration will be 1s22s22p63s23p4.
Using Electron Configurations
The configuration notation provides an easy way for scientists to write and communicate how electrons are arranged around the nucleus of an atom. It is also used to identify elements, and determine the chemical properties of elements and predict the behavior of atoms.
So, to answer the question, the atom with the electron configuration 1s2 2s2 2p6 3s2 3p4 is sulfur. Understanding electron configurations is an important concept in chemistry and can help you to identify elements, determine their chemical properties, and predict the behavior of atoms.
What is the electron configuration 1s2 2s2 2p3?
Do you ever wonder how electrons are arranged in an atom? Electron configuration is the term used to describe the distribution of electrons in an atom. It is a way of representing the arrangement of electrons in an atom in the form of a numerical expression. The electron configuration 1s2 2s2 2p3 is the arrangement of electrons in a nitrogen atom.
Understanding Electron Configurations
Electron configurations can be expressed in several ways. The most common way is to write out the orbital arrangement of electrons in an atom, with the orbitals listed in order of increasing energy. For example, the electron configuration of a nitrogen atom is 1s2 2s2 2p3. This means that the electrons in the nitrogen atom are arranged in the first shell (1s2), second shell (2s2), and third shell (2p3).
The number following the orbital letter indicates the number of electrons in that shell. In this example, there are two electrons in the first shell (1s2), two electrons in the second shell (2s2), and three electrons in the third shell (2p3). This adds up to a total of seven electrons in the nitrogen atom.
Valence Electrons
The electrons in the outermost shell of an atom are called valence electrons. In the example of the nitrogen atom, the valence electrons are the three electrons in the third shell (2p3). Valence electrons are important because they determine how an atom interacts with other atoms. These electrons are responsible for the chemical properties of an atom.
Is 1s2 2s2 2p6 Stable?
The electron configuration 1s2 2s2 2p6 is not a stable configuration. This is because nitrogen has an octet rule, which states that the outer shell of electrons should contain eight electrons. The 1s2 2s2 2p6 configuration has six electrons in the outer shell, which is two electrons short of the octet rule. Therefore, the most stable electronic configuration for nitrogen is 1s2 2s2 2p3.
What Are Electron Configurations?
Electron configurations are a way of describing the arrangement of electrons in an atom. They are written out in the form of a numerical expression, with the orbitals listed in order of increasing energy. The number following the orbital letter indicates the number of electrons in that shell. Valence electrons are the electrons in the outermost shell of an atom, and they are important for determining the chemical properties of an atom.
When n=4
When the principal quantum number (n) is equal to four, the subshells correspond to the angular momentum quantum numbers of l=0, l=1, l=2, and l=3. These subshells are named the s, p, d, and f subshells, respectively. The maximum number of electrons that can be accommodated by each subshell is given by the formula 2*(2l + 1). Therefore, the s, p, d, and f subshells can accommodate a maximum of two, six, ten, and fourteen electrons, respectively. All the possible subshells for values of n up to four are tabulated below.
Electron configurations are a way of describing the arrangement of electrons in an atom. The electron configuration 1s2 2s2 2p3 is the arrangement of electrons in a nitrogen atom. The number following the orbital letter indicates the number of electrons in that shell. Valence electrons are the electrons in the outermost shell of an atom, and they determine the chemical properties of an atom. When n=4, the subshells are named the s, p, d, and f subshells, and they can accommodate a maximum of two, six, ten, and fourteen electrons, respectively.
What element has the electron configuration 1s2 2s2 2p6 3s2 3p2 *?
Electron configurations are an important concept in chemistry, used to describe the arrangement of electrons within an atom. The electron configuration of an element tells us how electrons are distributed among the atomic orbitals. Knowing the electron configuration of an element is useful for determining its valency, predicting the properties of a group of elements, and interpreting atomic spectra.
What is an Electron Configuration?
An electron configuration is a notation that describes the arrangement of electrons within an atom. It is written in a standard format, which lists all electron-containing atomic subshells (with the number of electrons they hold written in superscript) in a sequence. For example, the electron configuration of sodium is 1s2 2s2 2p6 3s1.
Why is Knowing the Electron Configuration Important?
Knowing the electron configuration of an element can be useful for a variety of reasons. It can help determine the valency of the element, which is important for understanding how elements combine to form compounds. It can also be used to predict the properties of a group of elements with similar electron configurations, as elements with similar electron configurations tend to exhibit similar properties. Lastly, electron configurations can be used to interpret atomic spectra.
What is the Electron Configuration of 1s2 2s2 2p6 3s2 3p2 *?
The electron configuration 1s2 2s2 2p6 3s2 3p2 * is the electron configuration of an element with atomic number 13, which is aluminum. Aluminum contains 13 electrons, which are distributed as follows: 1s2 2s2 2p6 3s2 3p1. This is the correct answer.
What are the Incorrect Answers?
Option E is incorrect, as it corresponds to the electron configuration of sodium (atomic number 11). Sodium contains 11 electrons, which are arranged as follows: 1s2 2s2 2p6 3s1.
Option B is incorrect, as it corresponds to the electron configuration of neon (atomic number 10). Neon contains 10 electrons, which are arranged as follows: 1s2 2s2 2p6.
In conclusion, the electron configuration 1s2 2s2 2p6 3s2 3p2 * corresponds to the element aluminum (atomic number 13). Knowing the electron configuration of an element can be useful for determining its valency, predicting the properties of a group of elements, and interpreting atomic spectra.


Leave a Comment