Atoms are the building blocks of everything in the Universe and understanding them is key to understanding the world around us. When we think of atoms, we usually think of the number of protons, neutrons and electrons that make it up. But what about when we want to know what atoms have 24 protons and 21 neutrons? Or what has 20 protons and 20 neutrons atomic number? Or what atoms have 21 electrons?
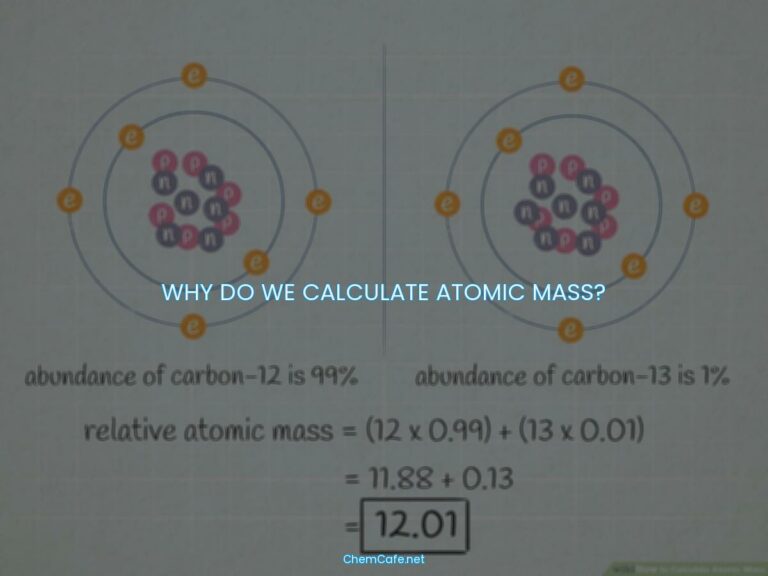
To answer these questions, it helps to understand the basics of isotopes. An isotope is any atom with a different number of neutrons than protons. All atoms of the same element have the same number of protons, but the number of neutrons can vary. This means that different isotopes of the same element can have different mass numbers. Isotopes are identified by their mass, which is the total number of protons and neutrons. There are two ways that isotopes are generally written. They both use the mass of the atom where mass = (number of protons) + (number of neutrons).
Using this information, you can determine which atoms have 24 protons and 21 neutrons. This is the chromium isotope with the mass number of 52, or Cr-52. All you need to know to answer this question is where chromium is on the periodic table. You will find that chromium’s atomic number is 24. This means that all chromium atoms have 24 protons in their nuclei. In a neutral atom, the number of protons is equal to the number of electrons. Therefore, all neutral chromium atoms, regardless of mass number, contain not only 24 protons in their nuclei, but also 24 electrons in their electron clouds.
It’s also possible to answer questions about other isotopes. For example, what has 37 protons in the nucleus? This is the element potassium, or K. Its atomic number is 37, meaning that all potassium atoms have 37 protons in their nuclei. Or, what element has 50 protons and 62 neutrons? This is the element tin, or Sn. All tin atoms have 50 protons in their nuclei and 62 neutrons.
Whether you are trying to understand the basics of isotopes or searching for answers to specific questions, understanding the number of protons and neutrons in different elements can help. So the next time you find yourself wondering what has 24 protons and 21 neutrons, you’ll know the answer!
What has 24 protons and 21 neutrons?
Isotopes are atoms of the same element that have different numbers of neutrons. They can be identified by their mass, which is the total number of protons and neutrons. There are two ways that isotopes are generally written. They both use the mass of the atom where mass = (number of protons) + (number of neutrons).
How Many Electrons are in Cr-52?
Chromium is a chemical element with the symbol Cr and atomic number 24. Its name comes from the Greek word ‘chroma’ meaning color. The isotope of chromium with the mass number of 52 is Cr-52. This means that its nucleus contains 24 protons and 28 neutrons.
All you need to know to answer the question of how many electrons are in Cr-52 is where chromium is on the periodic table. You will find that chromium’s atomic number is 24. This means that all chromium atoms have 24 protons in their nuclei.
In a neutral atom, the number of protons is equal to the number of electrons. Therefore, all neutral chromium atoms, regardless of mass number, contain not only 24 protons in their nuclei, but also 24 electrons in their electron clouds.
Unique Properties of Isotopes
The presence of isotopes in nature creates unique properties for each element. For example, different isotopes of carbon can be used to measure the age of organic material. This is known as carbon dating.
The unique properties of isotopes also allow scientists to trace the origin of certain elements. For example, scientists can trace the origins of nitrogen by studying the ratio of nitrogen-14 to nitrogen-15.
Uses of Isotopes
Isotopes have a variety of uses in the medical field. Radioactive isotopes are used in medical imaging to diagnose medical conditions such as cancer. They are also used in radiation therapy to treat certain types of cancer.
In addition, isotopes are used in research and development to study the structure and function of cells, organs and systems. They are also used to study the environment and are used in nuclear power plants to generate electricity.
In conclusion, isotopes are atoms of the same element that have different numbers of neutrons. They can be identified by their mass, which is the total number of protons and neutrons. Chromium is an element with the mass number of 52 and an atomic number of 24, meaning it has 24 protons and 28 neutrons. As a neutral atom, it has 24 electrons in its electron cloud. Isotopes have unique properties and are used in a variety of ways, including medical imaging, radiation therapy, and research and development.
What has 20 protons and 20 neutrons atomic number?
Atoms are the basic building blocks of all matter in the universe. They consist of sub-atomic particles such as electrons, protons, and neutrons, which all have different charges and masses. The number of protons and electrons in an atom is equal to its atomic number, while the number of neutrons can vary. The sum of the protons and neutrons contributes to the mass of an atom.
The atom having 20 protons and 20 neutrons has an atomic number of 20 and a mass number of 40. This means that the element is calcium, with the symbol Ca. Calcium is an alkaline earth metal located in group 2 of the periodic table.
What is the Atomic Number?
The atomic number of an element is the number of protons in the nucleus of each atom of the element. It is denoted by the symbol Z. The atomic number of an element determines its position in the periodic table, and it is also the number of electrons in a neutral atom.
What is the Mass Number?
The mass number of an element is the sum of the number of protons and neutrons in one atom of the element. It is denoted by the symbol A. The mass number of an element can vary with different isotopes, as the number of neutrons can differ.
What is the Symbol of an Element?
The symbol of an element is a one or two letter abbreviation that represents the element in the periodic table. For example, the symbol Ca stands for calcium, and the symbol H stands for hydrogen.
How to Find the Atom with 20 Protons and 20 Neutrons?
To find the atom with 20 protons and 20 neutrons, we must first consider the atomic number. The atomic number is the same as the number of protons in an atom. So, the element with 20 protons must be in the 20th column of the periodic table. This column contains the elements calcium and scandium.
Next, we must consider the mass number. The mass number is the sum of the protons and neutrons in an atom. Since the atom we are looking for has 20 protons and 20 neutrons, its mass number must be 40. This mass number is only found with the element calcium, so the atom with 20 protons and 20 neutrons is calcium, with the symbol Ca.
To recap, the atom with 20 protons and 20 neutrons has an atomic number of 20 and a mass number of 40. This means that the element is calcium, with the symbol Ca. Calcium is an alkaline earth metal located in group 2 of the periodic table. It is important to note that this atom can also have different isotopes, with varying numbers of neutrons.
Knowing how to find the atom with a certain number of protons and neutrons can be very useful in many fields. It is used in chemistry, physics, and engineering to understand the properties of different elements. It can also be used to identify unknown elements and compounds.
What atoms have 21 electrons?
Atoms are composed of electrons, protons, and neutrons. The number of electrons in an atom determines the element it belongs to. For example, the element sodium has 11 electrons, which gives it the atomic number 11. In general, each element has a characteristic number of electrons equal to its atomic number.
When an atom is electrically neutral, it must have the same number of protons and electrons. Thus, each element, at least when electrically neutral, has a characteristic number of electrons equal to its atomic number. An early model of the atom was developed in 1913 by Danish scientist Niels Bohr (1885–1962). In this model, electrons exist within principal shells. An electron normally exists in the lowest energy shell available, which is the one closest to the nucleus.
The Octet Rule
The innermost shell has a maximum of two electrons but the next two electron shells can each have a maximum of eight electrons. This is known as the octet rule, which states, with the exception of the innermost shell, that atoms are more stable energetically when they have eight electrons in their valence shell, the outermost electron shell. Examples of some neutral atoms and their electron configurations are shown in Figure 2.
What are Electron Configurations?
The electron configuration of an element describes how electrons are distributed in its atomic orbitals. Electron configurations of atoms follow a standard notation in which all electron-containing atomic subshells (with the number of electrons they hold written in superscript) are placed in a sequence. For example, the electron configuration of sodium is 1s22s22p63s1.
Atoms with 21 Electrons
Atoms with 21 electrons are in Group 3A on the periodic table. These elements are scandium (Sc), yttrium (Y), and lanthanum (La). Scandium is a silvery-white metal that is relatively rare in nature, while yttrium is a silvery-gray metal that is relatively abundant in the Earth’s crust. Lanthanum is a silvery-white metal that is relatively abundant in the Earth’s crust.
The electron configurations for these elements are as follows:
- Scandium: 1s22s22p63s23p63d14s2
- Yttrium: 1s22s22p63s23p63d14s24p64d1
- Lanthanum: 1s22s22p63s23p63d14s24p64d15s2
As can be seen, the outermost shell for each of these elements has three electrons, which is why they are in Group 3A on the periodic table.
Atoms with 21 electrons are in Group 3A on the periodic table and include scandium, yttrium, and lanthanum. The octet rule states that atoms are more stable energetically when they have eight electrons in their valence shell, the outermost electron shell. The electron configuration of an element describes how electrons are distributed in its atomic orbitals and is written using a standard notation.
Which atom has 21 electrons?
Atoms are composed of protons, neutrons, and electrons. The number of protons and electrons in an atom determines its chemical properties. Thus, each element, at least when electrically neutral, has a characteristic number of electrons equal to its atomic number.
This means that a chlorine atom, for example, has an atomic number of 17 and thus 17 electrons. But which atom has 21 electrons?
The Characteristics of Atoms
An early model of the atom was developed in 1913 by Danish scientist Niels Bohr (1885–1962). In this model, electrons exist within principal shells. An electron normally exists in the lowest energy shell available, which is the one closest to the nucleus.
Progressing from one atom to the next in the periodic table, the electron structure can be worked out by fitting an extra electron into the next available orbital. The closest orbital to the nucleus, called the 1s orbital, can hold up to two electrons. This orbital is equivalent to the innermost electron shell of the Bohr model of the atom. It is called the 1s orbital because it is spherical around the nucleus.
The innermost shell has a maximum of two electrons but the next two electron shells can each have a maximum of eight electrons. This is known as the octet rule, which states, with the exception of the innermost shell, that atoms are more stable energetically when they have eight electrons in their valence shell, the outermost electron shell. Examples of some neutral atoms and their electron configurations are shown in Figure 2.
Atom With 21 Electrons
The atom with 21 electrons is scandium (Sc). Scandium is a silvery-white metallic element that belongs to the transition metals in Group 3 of the periodic table. Its atomic number is 21, which means it has 21 protons and 21 electrons.
Scandium is located in the fourth period of the periodic table, and its electron configuration is 2-8-9-2. This means it has two electrons in the first shell, eight electrons in the second shell, nine electrons in the third shell, and two electrons in the fourth shell.
Uses of Scandium
Scandium is a relatively rare element, and it is mainly used in research and industrial applications. It is used as a catalyst in the production of polyolefins, which are a type of plastic. It is also used in the manufacture of fluorescent lamps and mercury-vapor lamps, and it is added to aluminum alloy to form a lightweight and strong material called scandium-aluminum.
Scandium is also used in the aerospace industry, where its lightweight properties make it ideal for the construction of aircraft and rocket fuel tanks. It is also used in sporting goods, such as baseball bats and golf clubs, as it can improve the performance of these items.
Atoms are composed of protons, neutrons, and electrons, and the number of protons and electrons in an atom determines its chemical properties. The atom with 21 electrons is scandium (Sc). Scandium is a relatively rare element, and it is mainly used in research and industrial applications. It is used as a catalyst in the production of polyolefins, in the manufacture of fluorescent lamps and mercury-vapor lamps, and it is added to aluminum alloy to form a lightweight and strong material. Scandium is also used in the aerospace industry and in sporting goods, such as baseball bats and golf clubs.
What has 37 protons in the nucleus?
Protons are positively charged particles that form the nucleus of an atom. The total number of protons in the nucleus of an atom is called the atomic number of the atom, and is given the symbol Z. The atomic number of an element is the same as the number of protons in its nucleus.
The 37th element on the periodic table is rubidium, and it has an atomic number of 37. This means that the nucleus of a rubidium atom contains 37 protons. Rubidium is a soft, silvery-white metallic element that is highly reactive, and it is used in various applications such as in the production of vacuums and as a catalyst for chemical reactions.
Protons are one of the three main components of an atom, along with electrons and neutrons. The total number of protons and neutrons in an atom is called its mass number, and is given the symbol A. The mass number of an element is the same as the number of protons and neutrons in its nucleus. Rubidium has a mass number of 87, which means that its nucleus contains 37 protons and 50 neutrons.
Protons have a rest mass of 1.67262 x 10-27 kg, which is nearly 1836 times greater than that of the electron. The diameter of a proton particle is about 2.4 x 10-13 cm. Protons are very important in nuclear reactions, as they act as the source of energy for nuclear fission and fusion reactions.
Protons are also important in chemistry, as they determine the chemical properties of an element. The number of protons in an atom determines the element’s identity, and it is this property that allows us to classify elements into groups on the periodic table. For example, all elements with an atomic number of 37 have similar properties and are classified as alkali metals.
Protons are also important in understanding atomic structure. The number of protons in an atom determines the charge on the nucleus, and this is what determines the attraction between the nucleus and the electrons. This is why elements with similar numbers of protons, such as rubidium (37 protons) and potassium (19 protons), have similar chemical properties.
In conclusion, the element rubidium has 37 protons in its nucleus. This is what gives it its atomic number of 37, and it is also what determines the element’s identity and its chemical properties. Protons are important in nuclear reactions and in understanding atomic structure, and they are essential for understanding the nature of matter.
What element has 50 protons and 62 neutrons?
When it comes to elements, isotopes are identified by their mass, which is the total number of protons and neutrons. The mass number of an isotope is the sum of the number of protons and neutrons, and is typically located below the element symbol on the periodic table.
An isotope with 50 protons and 62 neutrons would have a mass number of 112, and an actual mass of 1.90 x 10-25 kg. This means that, if the ions have lost just one electron back in the source S, they will carry a charge of 1.60 x 10-19 C.
Understanding Isotopes
Isotopes are atoms with the same number of protons, which makes them the same element, but with a different number of neutrons. This means that the mass of an isotope will be different than the mass of a regular atom.
The two ways that isotopes are generally written are both based on the mass of the atom. The formula for calculating the mass is [A=p^+ + n], where A is the mass number, p^+ is the number of protons, and n is the number of neutrons. Each proton and neutron has a mass of approximately one atomic mass unit (AMU).
Examples of Isotopes
Hydrogen is an example of an element with multiple isotopes. Hydrogen has three isotopes: Protium (H-1), Deuterium (H-2), and Tritium (H-3). Protium is the most common form of hydrogen, and it has only one proton and no neutrons. Deuterium has one proton and one neutron, and Tritium has one proton and two neutrons.
Carbon also has multiple isotopes. Carbon-12, or Carbon-12, has six protons and six neutrons. Carbon-13 has six protons and seven neutrons, and Carbon-14 has six protons and eight neutrons.
In conclusion, isotopes are atoms with the same number of protons but different numbers of neutrons, which makes them different elements. An isotope with 50 protons and 62 neutrons would have a mass number of 112, and an actual mass of 1.90 x 10-25 kg. This means that, if the ions have lost just one electron back in the source S, they will carry a charge of 1.60 x 10-19 C. Examples of elements with multiple isotopes include hydrogen and carbon.



Leave a Comment