Atomic mass is an important concept in chemistry and physics that can be used to understand the physical properties of an atom. It is the sum of the masses of the protons, neutrons, and electrons in an atom, or the average mass in a group of atoms. Atomic mass is used to differentiate different isotopes of elements, and is also important for determining the molar mass of a substance.
Atomic mass is a fundamental concept in chemistry and physics that can help us to understand the physical properties of an atom. It is the sum of the masses of the protons, neutrons, and electrons in an atom, or the average mass in a group of atoms. This is often expressed in unified atomic mass units, which is approximately one trillionth of one trillionth of a gram. By knowing the number of protons and neutrons in an atom, we can easily calculate the mass number of an atom by simply adding them together. This mass number is used to differentiate different isotopes of elements.
In addition to this, atomic mass is also important for determining the molar mass of a substance. Molar mass is the mass of one mole of a substance, which is equal to the atomic mass of the element in grams. This is used to calculate the amount of a substance that is needed in a reaction, and is an essential concept in chemistry.
Knowing atomic mass is also useful in other areas of science, such as physics, biology, and geology. For example, understanding the mass of atoms can help us to understand the behavior of particles and how they interact with each other. It can also be used to calculate the energy of a system and the forces between particles.
In summary, atomic mass is a fundamental concept in chemistry and physics that is used to understand the physical properties of an atom. It is used to differentiate different isotopes of elements and is also important for determining the molar mass of a substance. Knowing atomic mass is also important for many other areas of science and can help us to understand the behavior of particles and the forces between them.
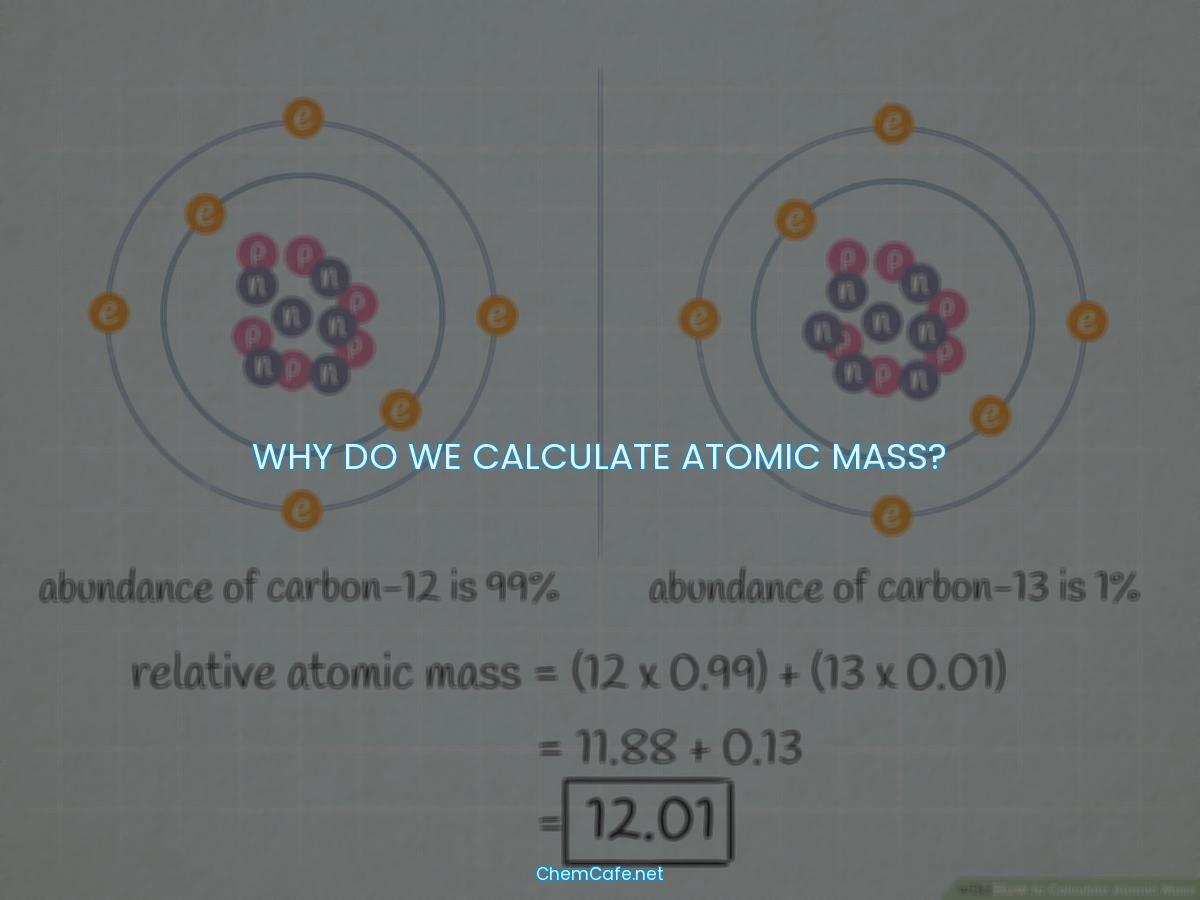
Why do we calculate atomic mass?
Atomic mass is an important concept in the world of chemistry and physics. It is used to differentiate between different isotopes of elements and to measure the atomic weight of atoms and molecules. But why do we need to calculate atomic mass?
Atomic mass is the sum of the masses of the protons, neutrons, and electrons in an atom or the average mass in a group of atoms. Since electrons have a much smaller mass than protons and neutrons, they are not included in the calculation. This means that the atomic mass is simply the sum of the masses of protons and neutrons.
What Is Atomic Mass?
Atomic mass is the total mass of the protons and neutrons in an atom. It is measured in unified atomic mass units (u) and is equal to one-twelfth of the mass of an atom of carbon-12. This number is also known as the atomic weight.
Atomic mass is an important concept in chemistry and physics. It is used to differentiate between different isotopes of elements, to measure the atomic weight of atoms and molecules, and to calculate the mass number of an atom.
Methods for Calculating Atomic Mass
There are three main methods for calculating atomic mass, depending on the information you have. The first one is to calculate the mass of a single atom. This requires knowledge of the number of protons and neutrons in the atom. The second method is to calculate the mass of a natural sample of the element. This requires knowledge of the relative abundance of the isotopes in the sample. The third method is to simply use the standard atomic mass of the element given in the periodic table.
Why Calculate Atomic Mass?
Atomic mass is important for a variety of reasons. It is used to determine the relative abundance of isotopes in a sample, to calculate the mass number of an atom, and to measure the atomic weight of atoms and molecules. It is also used to calculate the concentration of an element in a sample.
In addition, atomic mass is used to calculate the energy of an atom or molecule. This is because the mass of an atom is directly related to its energy. The more massive an atom is, the more energy it contains. This energy can then be released in the form of heat, light, or other forms of radiation.
In conclusion, atomic mass is an important concept in chemistry and physics. It is used to differentiate between different isotopes of elements, to measure the atomic weight of atoms and molecules, and to calculate the mass number of an atom. There are three main methods for calculating atomic mass, depending on the information you have. It is also used to calculate the concentration of an element in a sample and to calculate the energy of an atom or molecule. Knowing how to calculate atomic mass is essential for anyone working in the fields of chemistry and physics.
Why do we find atomic mass?
Atomic mass is an important concept in chemistry and physics. It is the total mass of all the protons, neutrons, and electrons in an atom or average mass of a group of atoms. Knowing atomic mass is necessary to calculate the number of atoms in a substance, the amount of energy released when atoms are combined, and the amount of energy released when atoms are split.
What is Atomic Mass?
Atomic mass is the sum of the masses of the protons, neutrons, and electrons in an atom, or the average mass of a group of atoms. It is expressed in atomic mass units (amu). One amu is equal to one-twelfth the mass of a single carbon-12 atom.
3 Ways to Find Atomic Mass
The method used to find atomic mass depends on whether you’re looking at a single atom, a natural sample, or a sample containing a known ratio of isotopes. Here are three methods for finding atomic mass:
1. Look up Atomic Mass on the Periodic Table
The first step in finding atomic mass is to look up the element in the periodic table. The atomic mass of the element is listed in the table as the atomic weight. However, keep in mind that electrons have so much less mass than protons and neutrons that they don’t factor into the calculation. So, the atomic mass is the sum of the masses of protons and neutrons.
2. Calculate Atomic Mass from Isotope Data
If you have data on the abundance of each isotope of an element, you can calculate the atomic mass by multiplying the mass of each isotope by its abundance and then adding up all the products. This method is used when you are dealing with a natural sample of the element.
3. Use Standard Atomic Mass Tables
The simplest way to find atomic mass is to look up the standard atomic mass of an element in a table. These tables, which are available online and in chemistry textbooks, list the atomic mass of each element, as well as the abundance of each isotope.
Atomic mass is an important concept in chemistry and physics. Knowing the atomic mass of an element or a sample is necessary for many calculations, such as calculating the number of atoms in a substance, the amount of energy released when atoms are combined, and the amount of energy released when atoms are split. There are three methods for finding atomic mass: looking up the atomic mass on the periodic table, calculating the atomic mass from isotope data, and using standard atomic mass tables.
Why is atomic weight important?
Atomic weight, also known as relative atomic mass, is an incredibly important concept in chemistry. It is the average mass of all the isotopes of an element, weighted by the abundance of each isotope. The atomic weight is used to calculate the mass of a single atom, and is essential for understanding the properties of a chemical element.
What is the difference between weight and mass?
Weight and mass are often used interchangeably, but they actually have different meanings. Weight is the measure of the force of gravity on an object, and is measured in newtons. Mass, on the other hand, is the measure of the amount of matter in an object, and is measured in kilograms. Although mass and weight are related, they are not the same.
What is the atomic mass unit (amu)?
The atomic mass unit (amu) is the standard unit used to measure the mass of atoms. It is equal to 1/12th of the mass of an atom of carbon-12 in its ground state. The amu was adopted as the standard unit in 1961, and is used to calculate the atomic weight of an element.
Can Atomic Mass and Atomic Weight Ever Be the Same?
Atomic mass and atomic weight can only be the same if the element only has one isotope. A pure element is composed of atoms that are all the same, and so the atomic mass and atomic weight will be the same. This is rare, however, as most elements have multiple isotopes.
Why is atomic number more important than atomic mass?
Atomic number is a more important concept than atomic mass because it tells us how many protons an element has. This number is essential for understanding the properties of an element, as it is the number of protons that determines the chemical properties of the element. The atomic mass, on the other hand, is the total mass of the protons, neutrons, and electrons in the atom, and does not directly affect the properties of the element.
In conclusion, atomic weight is an incredibly important concept in chemistry. It is the average mass of all the isotopes of an element, weighted by the abundance of each isotope. The atomic weight is used to calculate the mass of a single atom, and is essential for understanding the properties of a chemical element. Atomic number is more important than atomic mass because it tells us how many protons an element has, which is essential for understanding the properties of an element.
What is atomic mass and why is it important?
Atoms are the basic building blocks of matter, and their properties determine the chemical and physical nature of the elements. One of these properties is the atomic mass, which is the mass of an atom. Atomic mass is measured in Daltons, and is an essential element in chemists’ calculations.
Atomic mass is the sum of the masses of the individual particles that make up an atom. These particles include protons, which are positively charged, neutrons, which are neutral, and electrons, which are negatively charged. The protons and neutrons are located in the nucleus of the atom, while the electrons orbit around the nucleus.
What is the atomic mass unit?
The atomic mass unit, or u, is a unit of measurement used to calculate the mass of an atom or molecule. The atomic mass unit is defined as one-twelfth of the mass of a single atom of carbon-12. The atomic mass unit is used to calculate the average mass of the atoms of an element or of a molecule.
How is atomic mass calculated?
Atomic mass is calculated by adding the number of protons and neutrons in an atom. The number of protons and neutrons is determined by the atomic number of the element. The atomic number is the number of protons in the nucleus of an atom. The number of neutrons in an atom is equal to the atomic mass minus the atomic number.
What is the importance of atomic mass?
Atomic mass is important for a number of reasons. It is used to calculate the mass of elements and molecules, and to solve stoichiometry problems. Atomic mass is also used to calculate the amount of energy released or absorbed during a chemical reaction. Atomic mass is also used to calculate the average atomic mass of an element.
Atomic mass is an important property of matter, and it is used in a variety of calculations in chemistry. Atomic mass is calculated by adding the number of protons and neutrons in an atom, and it is measured in atomic mass units. Atomic mass is used to calculate the mass of elements and molecules, and to solve stoichiometry problems. Atomic mass is also used to calculate the amount of energy released or absorbed during a chemical reaction.
Why is relative atomic mass important?
In chemistry, it is important to quantify particles, such as atoms and molecules. But since the size of an atom is too small to be weighed practically, relative atomic mass (also known as relative atomic weight or atomic weight) is used to represent the mass or weight of an atom of an element.
Relative Atomic Mass Calculations
Relative atomic mass is a ratio of an element’s average atomic mass to one-twelfth of the mass of carbon-12. This ratio is typically abbreviated as r.a.m. The relative atomic mass of an element is given in the Periodic Table and is often used to calculate the mass of a compound or molecule.
The main difference between relative atomic mass and atomic mass is that relative atomic mass is a ratio, while atomic mass is the total mass of nucleons present in an atom’s nucleus. This makes relative atomic mass an important tool for chemists, as it allows them to easily compare the masses of different elements.
Why is it called relative atomic mass?
The term ‘relative atomic mass’ is used because it is the ratio of an element’s average atomic mass to one-twelfth of the mass of one carbon atom. This is important as it allows chemists to compare the masses of different elements in a consistent manner.
Relative atomic mass is also used to calculate the molar mass of a compound or molecule. This is because the molar mass of a substance is equal to the sum of the relative atomic masses of all the atoms in the molecule.
Relative atomic mass is an important tool for chemists, as it allows them to easily compare the masses of different elements. It is also used to calculate the molar mass of a compound or molecule, as the molar mass of a substance is equal to the sum of the relative atomic masses of all the atoms in the molecule.
Why do we calculate molar mass?
Molar mass is an important concept used in chemistry, helping us to understand the composition of chemical compounds and the amount of a substance in a given sample. Knowing the molar mass of a substance can help us to calculate the number of moles in a sample and understand the ratios of different elements in a compound. In this article, we’ll explore what molar mass is, how it’s calculated and why it’s so important.
What is Molar Mass?
Molar mass can be defined as ‘mass per mole.’ In other words, molar mass is the sum of the mass of all the atoms found in one mole’s worth of a substance. It is expressed in units of grams per mole. Molar mass is depicted for elements or molecules. In the case of single elements or individual atoms, the molar mass would just be the element’s mass expressed in atomic mass units.
Calculating Molar Mass
The molar mass is calculated in two ways. The first is through dimensional analysis, which involves multiplying the atomic mass of each element in a compound by the number of atoms of that element in the compound. This number is then divided by Avogadro’s number, which tells us the number of particles present per mole. The second method is to simply add up the atomic masses of all the elements in a compound.
Importance of Molar Mass
Molar mass is an important concept because it helps us to understand the amount of a substance in a given sample. Knowing the molar mass of a substance allows us to calculate the number of moles present in a sample, as well as the ratios of different elements in a compound. This can be helpful when calculating the mass of a substance, or when trying to understand the composition of a compound.
Molar mass is also important when it comes to understanding chemical reactions. Knowing the molar masses of reactants and products can help us to calculate the amount of energy released or absorbed during a reaction. This can help us to better understand the nature of a reaction and the conditions that are needed for it to occur.
Molar mass is an important concept in chemistry, helping us to understand the composition of chemical compounds and the amount of a substance in a given sample. Knowing the molar mass of a substance can help us to calculate the number of moles in a sample and understand the ratios of different elements in a compound. It can also help us to better understand chemical reactions, as knowing the molar masses of reactants and products can help us to calculate the amount of energy released or absorbed during a reaction.




Leave a Comment