Atoms are the fundamental building blocks of all matter, and every atom is comprised of three main particles: protons, neutrons, and electrons. Protons are positively charged, neutrons are uncharged, and electrons are negatively charged. Protons and neutrons have a mass of 1, while electrons have almost no mass. This balance between positive and negative charges is what gives atoms their unique properties.
But how are these particles arranged in an atom? What combination of protons, neutrons, and electrons make up a particular atom? To answer these questions, we must first understand the properties of these particles.
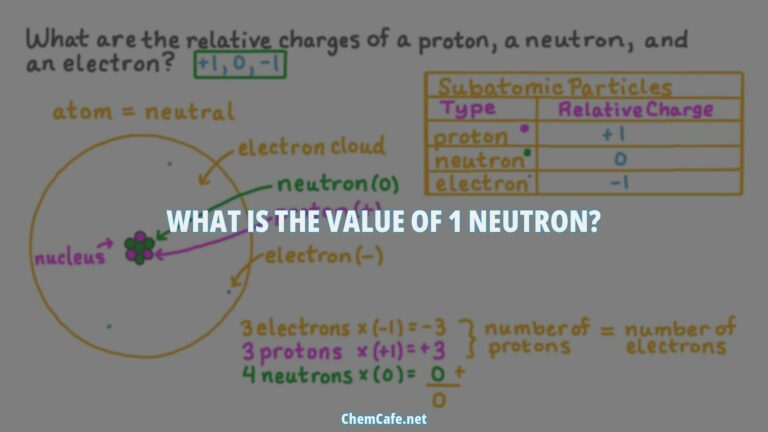
Protons have a charge of 1+ and a mass of 1 amu (atomic mass unit). Neutrons, on the other hand, have a mass of 1.0087 amu and a charge of zero, making them neutral. Electrons have a charge of 1- and are much lighter particles with a mass of about 0.00055 amu. These properties of these fundamental particles are summarized in Table 1.
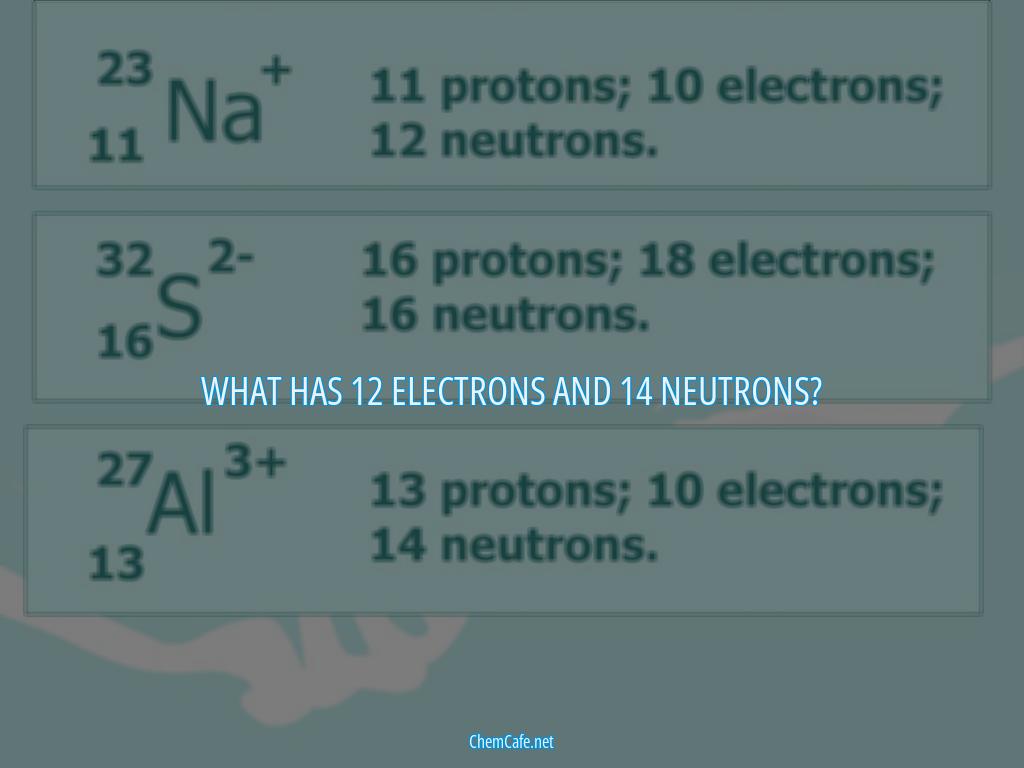
The atomic number of an element tells us how many protons are in its nucleus. For example, an atom of iodine (atomic number 53) contains 53 protons in its nucleus and 53 electrons outside its nucleus. Because the sum of the numbers of protons and neutrons equals the mass number, the number of neutrons can be determined. For example, a neutral iodine atom has a mass number of 127, so the number of neutrons is 74 (127 – 53 = 74).
This brings us to the question, what has 12 electrons and 14 neutrons? The answer is a chemical with 12 protons, 14 neutrons, and 10 electrons. This chemical would have an atomic number of 12, meaning that it contains 12 protons in its nucleus and 12 electrons outside its nucleus. The mass number of this chemical would be 26, so 14 neutrons would be present (26 – 12 = 14). This chemical would also have a charge of 2-, meaning that there would be 10 electrons present (12 – (2-) = 10).
In summary, atoms are made up of three fundamental particles: protons, neutrons, and electrons. Knowing the properties of these particles and the atomic number of an element, we can determine the number of protons, neutrons, and electrons in an atom. For example, a chemical with 12 electrons and 14 neutrons would have 12 protons, 14 neutrons, and 10 electrons.
What has 12 electrons and 14 neutrons?
Atoms are the building blocks of matter, and they are composed of three subatomic particles: protons, neutrons, and electrons. Protons are positively charged, neutrons are neutral, and electrons are negatively charged. The number of protons in an atom determines its atomic number and also its identity, while the number of neutrons can vary. So, what has 12 electrons and 14 neutrons?
The answer is iodine, a chemical element with atomic number 53. A neutral iodine atom contains 53 protons in its nucleus and 53 electrons outside its nucleus. Since the sum of the numbers of protons and neutrons equals the mass number, 127, the number of neutrons is 74 (127 − 53 = 74). When iodine is added as a 1− anion, the number of electrons is 54 [53 – (1–) = 54]. This means that an atom of iodine with 12 electrons and 14 neutrons has a total of 53 protons and 74 neutrons.
Properties of Electrons, Protons, and Neutrons
The properties of electrons, protons, and neutrons are summarized in Table (PageIndex{1}). A neutron is a slightly heavier particle with a mass of 1.0087 amu and a charge of zero; as its name suggests, it is neutral. The electron has a charge of 1− and is a much lighter particle with a mass of about 0.00055 amu (it would take about 1800 electrons to equal the mass of one proton).
Protons and neutrons are both found in the nucleus of an atom, while electrons are found in the electron cloud around the nucleus. Electrons are much smaller than protons and neutrons, so they are able to move around the nucleus at much higher speeds. This allows them to form bonds with other atoms and molecules, which is the basis of chemistry.
The Structure of Atoms
Atoms are made up of three main parts: a nucleus, an electron cloud, and a valence shell. The nucleus contains the protons and neutrons, while the electron cloud is composed of the electrons. The valence shell is the outermost layer of electrons and determines the chemical properties of the atom. For example, the valence shell of an atom of sodium is composed of one electron, so sodium is a highly reactive metal.
The electrons in the valence shell are held in place by the electromagnetic force, which is the same force that holds together protons and neutrons in the nucleus. The force of attraction between the negatively charged electrons and the positively charged protons is what gives an atom its overall neutral charge. This is why an atom with the same number of protons and electrons is electrically neutral.
In conclusion, atoms are composed of three subatomic particles: protons, neutrons, and electrons. An atom with 12 electrons and 14 neutrons has a total of 53 protons and 74 neutrons, and is an atom of iodine with an atomic number of 53. The properties of these particles are summarized in Table (PageIndex{1}). The electrons in the valence shell are held in place by the electromagnetic force, which is the same force that holds together protons and neutrons in the nucleus. This is why an atom with the same number of protons and electrons is electrically neutral.
What element has 12 protons 13 neutrons and 12 electrons?
It is not possible for an atom to have 12 protons and 13 electrons. This is because for a neutral atom, the number of protons must equal the number of electrons. Although it is possible for an atom to have 12 protons, 13 neutrons and 12 electrons, it would not be a neutral atom.
What is an Atom?
Atoms are the basic building blocks of matter. They are made up of three subatomic particles: protons, neutrons and electrons. Protons have a positive charge, neutrons have no charge, and electrons have a negative charge. The number of protons in an atom determines its identity, while the number of neutrons and electrons can vary depending on the isotope.
Atomic Number and Mass Number
The atomic number (Z) of an atom is equal to the number of protons in its nucleus. The mass number (A) is equal to the number of protons plus the number of neutrons in its nucleus. Isotopes are atoms that contain the same number of protons, but different numbers of neutrons. The mass number of an isotope is always the same, but the atomic number can vary depending on the number of neutrons.
Neutral Atom
A neutral atom must contain the same number of positive and negative charges, so the number of protons equals the number of electrons. If an atom has 12 protons and 12 electrons, it is a neutral atom. However, if it has 12 protons and 13 electrons, it is not a neutral atom.
Examples of Neutral Isotopes
There are several examples of neutral isotopes. Tin-120 is a neutral isotope that contains 50 protons, 50 electrons, and 70 neutrons. Its mass number is 120, while its atomic number is 50. Its isotope symbol is 120Sn.
Another example is an atom with a mass number (A) of 33 and an atomic number (Z) of 16. The element X in this atom is sulfur (S).
Finally, an atom with a mass number of 58 and 30 neutrons contains element X as chlorine (Cl).
It is not possible for an atom to have 12 protons, 13 neutrons and 12 electrons. This is because for a neutral atom, the number of protons must equal the number of electrons. However, it is possible for an atom to have 12 protons, 13 neutrons and 12 electrons, but it would not be a neutral atom. There are several examples of neutral isotopes, such as tin-120 and an atom with a mass number of 33 and an atomic number of 16.
What chemical has 12 protons 14 neutrons and 10 electrons?
Atoms are composed of three different subatomic particles: protons, neutrons, and electrons. Protons are positively charged, neutrons are neutral, and electrons are negatively charged. The number of each of these particles determines an atom’s identity and its chemical properties.
So, what chemical has 12 protons, 14 neutrons, and 10 electrons? This atom is Carbon-24. Carbon-24 is a stable isotope of the element carbon, which is the basis of all life on Earth. It is found naturally in trace amounts in the atmosphere and in the Earth’s crust.
Carbon-24 is composed of six protons, six neutrons, and six electrons. Its nucleus is composed of 12 protons and 14 neutrons, and its outer shell is composed of 10 electrons. The 12 protons give carbon-24 its identity as an element, and the 14 neutrons stabilize the atom. Without the 14 neutrons, the atom would be unstable and unable to form the complex molecules necessary for life.
The 10 electrons in the outer shell of the atom are responsible for its chemical properties. The electrons are arranged in two shells, with the first shell containing two electrons, and the second shell containing eight electrons. This arrangement gives the atom its characteristic reactivity, allowing it to form bonds with other atoms and molecules.
The stability of carbon-24 makes it an important part of living organisms, as it is the basis for the entire carbon cycle. Carbon-24 is essential for photosynthesis, the process by which plants turn sunlight into food. It is also a key component of proteins, the building blocks of life, and of DNA, the genetic material that determines our physical characteristics.
In summary, the chemical with 12 protons, 14 neutrons, and 10 electrons is carbon-24. This stable isotope of carbon is essential for life on Earth, as it is the basis of the carbon cycle, photosynthesis, proteins, and DNA. Without it, life as we know it would not exist.
What has 12 neutrons and 12 electrons?
Atoms and molecules can have different numbers of protons, neutrons, and electrons. When atoms have the same number of protons and electrons, they are considered neutral. The number of neutrons in an atom or molecule can vary, however, and this affects its properties. So, what has 12 neutrons and 12 electrons?
The answer is that there are a few different types of atoms and molecules that have 12 neutrons and 12 electrons. These include the argon atom, the free neutron, the cobalt atom, the hydrogen atom, and the helium atom. Let’s take a closer look at each of these.
40Ar – 18 protons, 22 neutrons, 18 electrons
The argon atom has 18 protons, as seen in the periodic table. There are also 18 electrons because the atom is neutral. There are 22 neutrons because 40 – 18 = 22.
n – 0 protons, 1 neutrons, 0 electrons
This is a free neutron, denoted by the lower case n. It has no protons and no electrons, but it does have 1 neutron.
60Co – 27 protons, 33 neutrons, 27 electrons
The cobalt atom has 27 protons, as seen in the periodic table. There are also 27 electrons because the charge is 0. There are 33 neutrons because 60 – 27 = 33.
3H – 1 protons, 2 neutrons, 1 electrons
The hydrogen atom has 1 proton, as seen in the periodic table. There is 1 electron because the atom is neutral. There are two neutrons because 3 – 1 = 2.
4He – 2 protons, 2 neutrons, 0 electrons
The helium atom has two protons, as seen in the periodic table. There are no electrons because 4 – 2 = 0. There are 2 neutrons because 4 – 2 = 2.
35Cl- – 17 protons, 18 neutrons, 18 electrons
This is a chloride ion. According to the periodic table, there are 17 protons because the element is chlorine. There are 18 electrons due to the negative charge: 17-(-1) = 18. There are 18 neutrons because 35 – 17 = 18.
In conclusion, there are a few different types of atoms and molecules that have 12 neutrons and 12 electrons. These include the argon atom, the free neutron, the cobalt atom, the hydrogen atom, and the helium atom. Each of these has its own unique set of properties that make it different from the others. Understanding the number of protons, neutrons, and electrons in each atom or molecule can help us better understand their properties and how they interact with each other.
What has 12 protons and 12 neutrons and 12 electrons?
Atoms are the fundamental building blocks of all matter in the universe. They are composed of three subatomic particles: protons, neutrons, and electrons. Each atom has a unique atomic number, which is the number of protons it contains.
The atom with 12 protons, 12 neutrons, and 12 electrons is Carbon-12, or 12C. It is the most abundant of all the isotopes of carbon, accounting for almost 99% of the carbon found in nature. Carbon-12 is a stable isotope, meaning that it does not undergo radioactive decay.
A proton has a positive charge, a neutron is neutral, and an electron has a negative charge. In a neutral atom, the number of protons and electrons must be equal, and the number of neutrons can vary. Carbon-12 has six protons and six electrons, and its number of neutrons is equal to its atomic number (12).
The protons and neutrons are found in the nucleus of the atom, and the electrons orbit the nucleus in shells. Carbon-12 has two electrons in its first shell, four in its second, and six in its third.
Other elements with 12 protons and 12 neutrons include Magnesium-24 (24Mg) and Manganese-24 (24Mn). Magnesium-24 is a stable isotope, meaning it does not undergo radioactive decay. It is found in the Earth’s crust and has many industrial and medical applications. Manganese-24 is a radioactive isotope with a half-life of 5.5 hours, meaning that half of the atoms will decay in that time. It is used in medical imaging and radiotherapy to diagnose and treat cancer.
If an atom has fewer than 12 protons, it is not carbon-12. For example, the isotope carbon-11 has 11 protons, 12 neutrons, and 11 electrons. It is a radioactive isotope with a half-life of 20.33 minutes. It is used in medical imaging to detect tumors and measure blood flow.
Atoms with more than 12 protons also exist. For example, the isotope Cobalt-60 (60Co) has 27 protons, 33 neutrons, and 27 electrons. It is a radioactive isotope with a half-life of 5.27 years. It is used in radiotherapy to treat cancer and has many industrial applications.
Finally, the isotope Hydrogen-3 (3H) has 1 proton, 2 neutrons, and 1 electron. It is a radioactive isotope with a half-life of 12.32 years. It is used in radiotherapy to treat tumors and in medical imaging to study metabolic processes.
In summary, Carbon-12 is an atom with 12 protons, 12 neutrons, and 12 electrons. It is a stable isotope that does not undergo radioactive decay. Other atoms with 12 protons and 12 neutrons include Magnesium-24 and Manganese-24. Atoms with fewer or more protons have different properties and applications.
What element has 12 protons 12 neutrons and 12 electrons?
Atoms of an element are identified by their atomic number, which is the number of protons they contain. The most common and stable type of atom found in nature is the one with an atomic number of 12, which is magnesium. This type of magnesium atom has 12 protons, 12 neutrons, and 12 electrons.
What are Protons and Neutrons?
Protons and neutrons are subatomic particles that make up the nucleus of an atom. Protons have a positive electric charge and neutrons have no electric charge. The number of protons in the nucleus of an atom determines its atomic number, while the number of neutrons can vary.
Why is the most stable type of magnesium atom 12 protons, 12 neutrons, and 12 electrons?
The combination of 12 protons, 12 neutrons, and 12 electrons is the most stable type of magnesium atom because it is the most balanced. The 12 protons give the atom its atomic number, while the 12 electrons give it a neutral charge. The 12 neutrons give the atom its mass, while also helping to maintain the balance of the atom.
What other elements have 12 protons, 12 neutrons, and 12 electrons?
Other elements that have 12 protons, 12 neutrons, and 12 electrons are carbon, oxygen, and iron. Carbon has 6 protons and 6 neutrons, while oxygen has 8 protons and 8 neutrons. Iron has 26 protons and 26 neutrons. These elements also have 12 electrons, which give them a neutral charge.
What other elements have different numbers of protons, neutrons, and electrons?
Other elements can have different numbers of protons, neutrons, and electrons. For example, chlorine has 17 protons, 18 neutrons, and 18 electrons. Chlorine has a negative charge due to the 18 electrons. Cobalt has 27 protons, 33 neutrons, and 27 electrons, while hydrogen has 1 proton, 2 neutrons, and 1 electron.
Atoms of an element are identified by their atomic number, which is the number of protons they contain. The most common and stable type of atom found in nature is the one with an atomic number of 12, which is magnesium. This type of magnesium atom has 12 protons, 12 neutrons, and 12 electrons. Other elements that have 12 protons, 12 neutrons, and 12 electrons are carbon, oxygen, and iron. Other elements can have different numbers of protons, neutrons, and electrons, such as chlorine, cobalt, and hydrogen. This “exotic species” of atoms is highly sought after and is a crucial part of understanding the periodic table.



Leave a Comment