Have you ever heard of isotopes? These are variants of the same chemical element that have the same atomic number but different numbers of neutrons in the nucleus. The discovery of isotopes marked a major breakthrough in Dalton’s atomic theory which was the first theory to describe the structure of atoms. Every element has one or more isotopes, and they can be identified by a notation known as A/Z or symbol-mass format.
When scientists discuss individual isotopes, they need an efficient way to specify the number of neutrons in any particular nucleus. That is why the A/Z or symbol-mass format was developed. This system uses a combination of the atomic number, the symbol of the element, and the mass number of the isotope to uniquely identify it. With this notation, scientists can easily identify the isotopes of any element.
Isotopes have a variety of uses, from medical to industrial applications. In medicine, they are used in radiotherapy and positron emission tomography (PET). In industry, isotopes are used to measure the age and origin of materials. Some isotopes are even used as tracers to study the movement of materials in the environment.
Clearly, the ability to identify and distinguish between isotopes is essential for a variety of research applications. Thus, understanding how to identify the isotope of an element is extremely important. A/Z and symbol-mass formats can be used to accurately display the information and to differentiate between different isotopes. By understanding how these formats work and how to use them, scientists can easily identify the isotopes of any element.
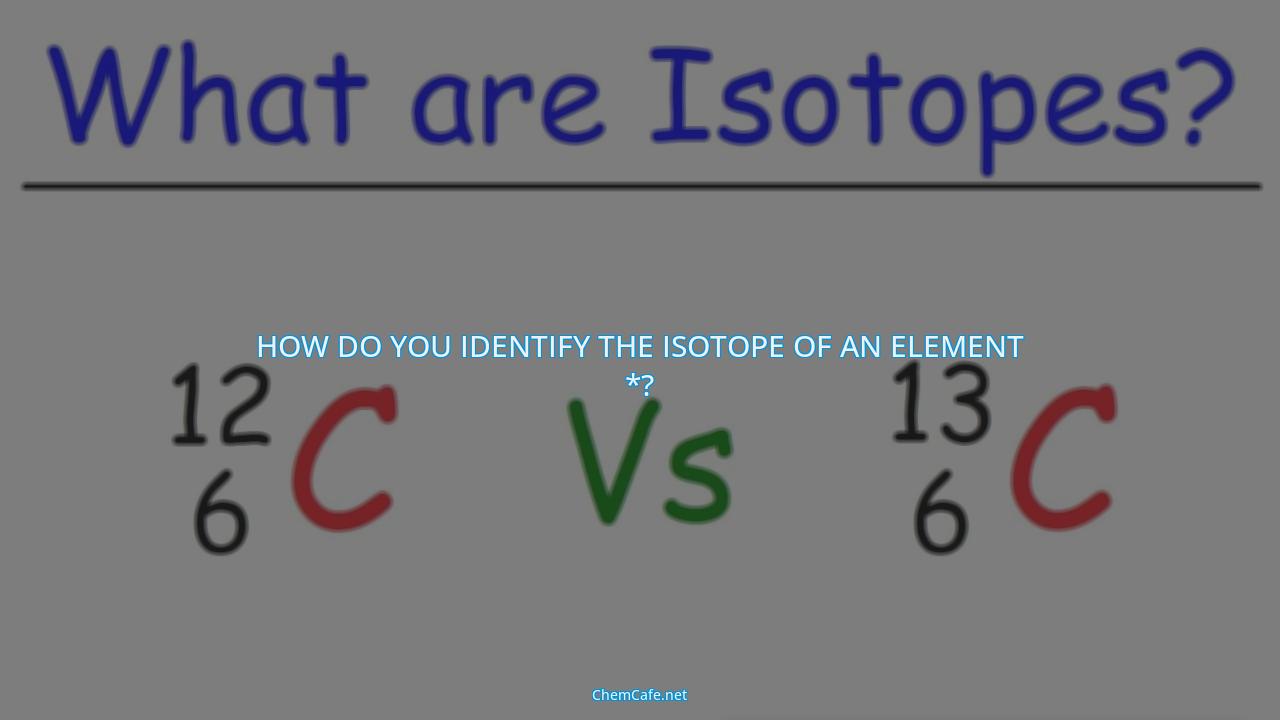
How do you identify the isotope of an element *?
Atoms of a given element can have different numbers of neutrons in their nuclei, resulting in different isotopes of that element. Identifying the isotope of an element helps scientists in understanding the properties of the element and its behavior in different environments.
Dalton’s Atomic Theory
The discovery of isotopes required a minor change in Dalton’s atomic theory. Dalton’s atomic theory postulated that all atoms of an element were identical and had the same mass. This theory was challenged when it was found that atoms of the same element could have different masses.
Atomic Number
An atom is first identified and labeled according to the number of protons in its nucleus. This atomic number is ordinarily given the symbol Z. The great importance of the atomic number derives from the observation that all atoms with the same atomic number have nearly, if not precisely, identical chemical properties. A large collection of atoms with the same atomic number constitutes a sample of an element.
Isotope Notation
An isotope of any element can be uniquely represented as ({}_Z^{A}X)where X is the atomic symbol of the element. The superscript A denotes the mass number, which is equal to the total number of protons and neutrons in the nucleus. The subscript Z is the atomic number, which is equal to the number of protons in the nucleus.
Symbol-Mass Format
When scientists discuss individual isotopes, they need an efficient way to specify the number of neutrons in any particular nucleus. A/Z and symbol-mass formats can be used to display periodic table information. When viewing either of these two notations, isotopic differences can be obtained.
Every chemical element has one or more isotopes. By using the atomic number and mass number, scientists can identify and label the isotope of any element. This helps in understanding the properties of the element and its behavior in different environments. With the help of isotope notation, scientists can efficiently specify the number of neutrons in any particular nucleus.
How do you identify isotopes?
Isotopes are atoms of the same element that have different numbers of neutrons. This difference in neutron amount affects the atomic mass (A) but not the atomic number (Z). The discovery of isotopes required a minor change in Dalton’s atomic theory. Currently, there are over 3,500 isotopes known for all the elements.
Identifying isotopes is an important task for scientists, as it can give us greater insight into the properties and behavior of atoms. There are two main ways to identify isotopes. The first is by mass, which is the total number of protons and neutrons. The second is by the notation used to display periodic table information.
Mass of an Isotope
The mass of an isotope can be expressed as mass = (number of protons) + (number of neutrons). For example, the isotope of hydrogen with one proton and one neutron has a mass of 2. This is the same for any other element, where the mass of an isotope is determined by the number of protons and neutrons it contains.
Notations Used to Display Periodic Table Information
When scientists discuss individual isotopes, they need an efficient way to specify the number of neutrons in any particular nucleus. A/Z and symbol-mass formats (refer to Section 3.4) can be used to display periodic table information. When viewing either of these two notations, isotopic differences can be obtained.
For example, the notation for a carbon-14 isotope is 14C. This is because it has six protons and eight neutrons, giving it a mass of 14. The same can be done for other elements, where the element symbol is followed by the mass of the isotope.
Separating Different Types of Atoms
In a chemical laboratory, isotopes of an element appear and react the same. For this reason, it is difficult to distinguish between an atom’s isotopes. In contrast, nuclear scientists can identify and separate different types of atomic nuclei (Figure (PageIndex{1})).
The process of separating isotopes is known as isotope separation. This can be done using a variety of methods, including mass spectrometry, centrifugation, and fractional distillation. By using these methods, scientists can identify and separate different types of atomic nuclei.
Isotopes can be identified by their mass, which is the total number of protons and neutrons. There are two ways that isotopes are generally written. They both use the mass of the atom where mass = (number of protons) + (number of neutrons). The discovery of isotopes required a minor change in Dalton’s atomic theory. In a chemical laboratory, isotopes of an element appear and react the same. For this reason, it is difficult to distinguish between an atom’s isotopes. Nuclear scientists, however, can identify and separate different types of atomic nuclei using methods such as mass spectrometry, centrifugation, and fractional distillation.
How do you identify an isotope?
Isotopes are forms of the same element, but with different numbers of neutrons. They are identified by their mass, which is the total number of protons and neutrons. There are two main ways that isotopes are written, and both use the mass of the atom.
Mass = (number of protons) + (number of neutrons)
The first method is Standard Notation, also known as AZE notation. In this format, A is the mass number, Z is the atomic number, and E is the element symbol. The mass number “A” is indicated with a superscript to the left of the chemical symbol “E” while the atomic number “Z” is indicated with a subscript. For example, the isotope of carbon with mass number 14 and atomic number 6 can be written as 14C.
Periodic Table Notation
The second method of writing isotopes is by using the Periodic Table Notation. This notation displays the periodic table information in a table format. Here, the element symbol is listed in the left column, followed by the mass number and atomic number in the right columns. For example, the isotope of carbon with mass number 14 and atomic number 6 can be written as C-14-6.
Isotopic Differences
When viewing either the AZE or Periodic Table notation, isotopic differences can be obtained. Isotopic differences are the differences in mass numbers between two isotopes of the same element. For example, if two isotopes of carbon were being compared, the difference in mass numbers would be 14 – 12 = 2. This difference indicates that there are two more neutrons in the 14C isotope than in the 12C isotope.
Discovery of Isotopes
The discovery of isotopes required a minor change in Dalton’s atomic theory. Before the discovery of isotopes, Dalton’s atomic theory stated that all atoms of the same element have the same mass. However, scientists found that atoms of the same element could have different masses, and thus the discovery of isotopes was made.
Identifying isotopes can help scientists understand the properties of elements and their compounds. By understanding the properties of isotopes, scientists can develop new technologies and treatments for diseases, as well as gain insight into the formation of our universe. Isotopes are an important part of the study of chemistry, and are essential for furthering our understanding of the elements and the universe around us.
How do you find the isotopes of an element?
Isotopes are atoms that have the same number of protons, but different numbers of neutrons. They are the same element but with different masses, and they can be found on the periodic table. Identifying isotopes can be tricky, but once you understand how to read the periodic table and find the atomic number and mass, it’s a relatively simple process.
Find the Element
Start by finding the element you’re looking for on the periodic table. Each element is represented by a box with its chemical symbol and atomic number on the top left corner. The atomic number is the number of protons in the nucleus of the atom.
Calculate the Mass
Once you have the atomic number, you can calculate the mass of the atom. To do this, you must add the number of protons to the number of neutrons in the nucleus. This number can be found on the bottom of the box in the periodic table. The number of neutrons will be the same for all isotopes of the same element.
Identify the Isotopes
Once you know the mass of the atom, you can identify the isotopes. Isotopes are usually written in one of two ways: as the atomic number followed by the mass (e.g. 12C-14C) or as the mass followed by the atomic number (e.g. 14C-12C). In either case, the first number is the number of protons, and the second number is the total number of protons and neutrons.
Finding the Most Common Isotope
The most common isotope of an element is usually the one with the most neutrons. To find the most common isotope, look up the element’s atomic number and mass on the periodic table. The isotope with the most neutrons will have the greatest mass.
Finding the isotopes of an element is not difficult, once you know how to read the periodic table. All you need to do is look up the atomic number and mass of the element, then use that information to identify the isotopes. Knowing how to find isotopes can be useful for a variety of applications, from isotope dating to nuclear medicine.
How do you find the isotope of an element?
Isotopes are different forms of an element that have the same number of protons but different numbers of neutrons. They are identified by their mass number, which is the total number of protons and neutrons. Finding the isotope of an element can be done in two ways.
Method 1: Using Atomic Number
The atomic number of an element denotes the number of protons in the nucleus, and thus it is the same for all isotopes of an element. To find the isotope of an element, you need to first identify the element’s atomic number. This can be done by looking up the element’s name in the periodic table of the elements. Once you have identified the atomic number, you can use the Periodic Table of Isotopes to find the corresponding isotopes. The Periodic Table of Isotopes lists the isotopes of each element in order of their atomic number.
Method 2: Using Mass Number
The mass number of an element denotes the total number of protons and neutrons in the nucleus. To find the isotope of an element, you need to first identify the element’s mass number. This can be done by looking up the element’s name in the periodic table of the elements. Once you have identified the mass number, you can use the Periodic Table of Isotopes to find the corresponding isotopes. The Periodic Table of Isotopes lists the isotopes of each element in order of their mass number.
In summary, isotopes are atoms of the same element that have the same number of protons but different numbers of neutrons. To find the isotope of an element, you can either use the element’s atomic number or its mass number. The Periodic Table of Isotopes can be used to find the isotopes of an element, as it lists the isotopes of each element in order of their atomic or mass number. Understanding the concept of isotopes is essential to the study of chemistry, as it helps to explain the behavior of atoms and molecules.
How do you find isotopes?
Isotopes are atoms of the same element that have a different number of neutrons. This can be seen in the periodic table, which divides elements into columns based on the number of protons in their nucleus. Each element has a different number of isotopes, and it is important to be able to identify them. Here is a guide to finding isotopes and understanding the notation used to represent them.
A/Z and Symbol-Mass Notation
In order to identify isotopes, scientists need an efficient way to specify the number of neutrons in any particular nucleus. This is where the A/Z and symbol-mass notation comes in. A/Z notation is a way of writing a nucleus of an atom, with the ‘A’ representing the atomic number (the number of protons) and the ‘Z’ representing the atomic mass (the number of protons and neutrons).
For example, nitrogen-14 (14N) is written as 14/7N. Symbol-mass notation is similar, but it uses the atomic mass instead of the atomic number. For nitrogen-14, it would be written as 14N.
Finding the Most Common Isotope
To find the most common isotope of an element, you first need to find the element on the periodic table. You can then get the atomic number (the number of protons) from the top left corner of the box and the atomic weight (the number of neutrons) from the bottom of the box.
For example, hydrogen has an atomic number of 1 and an atomic weight of 1.008. This means that the most common isotope of hydrogen is protium, which has one proton and no neutrons.
Discovering Isotopes
The discovery of isotopes also required a minor change in Dalton’s atomic theory. Dalton’s theory stated that all atoms of an element had identical properties, so the discovery of isotopes meant that atoms of the same element could have different masses. This led to the development of the atomic mass unit (amu), which is used to measure the mass of atoms.
In conclusion, isotopes are identified by their mass, which is the total number of protons and neutrons. There are two ways that isotopes are generally written – A/Z and symbol-mass notation. The most common isotope of an element can be found by looking at the atomic number and atomic weight of the element on the periodic table. The discovery of isotopes also required a minor change in Dalton’s atomic theory. With this guide, you should now be able to identify isotopes and understand the notation used to represent them.




Leave a Comment